Abstract
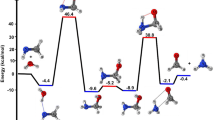
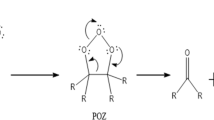
Ab initio calculations at the G2 level were used in a theoretical analysis of the kinetics of the decomposition of trifluoro-, trichloro-, and tribromomethanols. The high-pressure limiting rate coefficients kdiss,∞ for the thermal dissociation of CF3OH, CCl3OH, and CBr3OH were calculated using the conventional transition state theory. The results of potential surface calculations show that in the presence of the hydrogen halides HX (X = F, Cl, and Br), considerably lower energy pathways are accessible for the decomposition of CF3OH, CCl3OH, and CBr3OH. The mechanism of the reactions appears to be complex and consists of three consecutive elementary processes with the formation of pre- and post-reaction adducts. The presence of hydrogen halides considerably decreases the energy barrier for the bimolecular decomposition of the alcohols CF3OH, CCl3OH, and CBr3OH. Results of this study indicate that hydrogen halides can considerably accelerate the homogeneous decomposition of perhalogenated methanols when they are present in the reaction area at sufficiently high concentrations. However, the atmospheric concentrations of hydrogen halides are too small for efficient removal of atmospheric CF3OH, CCl3OH, and CBr3OH.




Similar content being viewed by others
References
Finnlayson-Pitts BJ, Pitts JN (2000) Chemistry of the Upper and Lower Atmosphere. Academic, San Diego
Wayne RP, Poulet G, Biggs P, Burrows JP, Cox RA, Crutzen PJ, Hayman GD, Jenkin ME, LeBras G, Moortgat GK, Platt U, Schindler RN (1995) Atmos Environ 29:2677–2881
Wallington TJ, Dagaut P, Kurylo MJ (1992) Chem Rev 92:667–710
Sehested J, Wallington TJ (1993) Environ Sci Technol 27:146–152
Wallington TJ, Schneider WF (1994) Environ Sci Technol 28:1198–1200
Schneider WF, Wallington TJ, Minschwaner K, Stahlberg EA (1995) Environ Sci Technol 29:247–250
Huey LG, Hanson DR, Lovejoy ER (1995) J Geophys Res 100:18771–18774
Bednarek G, Kohlmann JP, Saathoff H (1995) Zellner R. Z Phys Chem Munich 188:1–15
Lovejoy ER, Huey LG, Hanson DR (1995) J Geophys Res 100:18775–18780
Wallington TJ, Hurley MD, Schneider WF, Sehested J, Nielsen OJ (1993) J Phys Chem 97:7606–7611
Francisco JS (1991) Chem Phys 150:19–27
Bock CW, Trachtman M, Niki H, Mains GJ (1994) J Phys Chem 98:7976–7980
Francisco JS (1994) Chem Phys Lett 218:401–405
Schneider WF, Wallington TJ, Huie RE (1996) J Phys Chem 100:6097–6103
Kim SJ, Song HS (1999) Bull Korean Chem Soc 20:1493–1500
Brudnik K, Jodkowski JT, Ratajczak E, Venkatraman R, Nowek A, Sullivan RH (2001) Chem Phys Lett 345:435–444
Brudnik K, Jodkowski JT, Ratajczak E (2003) J Mol Struct 656:333–339
Brudnik K, Jodkowski JT, Ratajczak E (2003) Bull Pol Acad Sci Chem 51:77–91
Fernández LE, Varetti EL (2003) J Mol Struct THEOCHEM 629:175–183
Tyndall GS, Wallington TJ, Hurley MD, Schneider WF (1993) J Phys Chem 97:1576–1582
Wallington TJ, Schneider WF, Barnes I, Becker KH, Sehested J, Nielsen OJ (2000) Chem Phys Lett 322:97–102
Schnell M, Mühlhäuser M, Peyerimhoff SD (2002) Chem Phys Lett 361:1–7
Sun H, Bozzelli JW (2001) J Phys Chem A 105:4504–4516
Brudnik K, Jodkowski JT, Nowek A, Leszczynski J (2007) Chem Phys Lett 435:194–200
Montgomery JA, Michels HH, Francisco JS (1994) Chem Phys Lett 220:391–396
Notario R, Castaño O, Abboud JLM (1996) Chem Phys Lett 263:367–370
Espinosa-Garcia J (1999) Chem Phys Lett 315:239–247
Segovia M, Ventura ON (1997) Chem Phys Lett 277:490–496
Brudnik K, Wójcik-Pastuszka D, Jodkowski JT, Leszczynski J (2008) J Mol Model 14:1159–1172
Vöhringer-Martinez E, Hansmann B, Hernandez H, Francisco JS, Troe J, Abel B (2007) Science 315:497–501
Garrett BC (2004) Science 303:1146–1147
Takahashi K, Kramer ZC, Vaida V, Skodje RT (2007) Phys Chem Chem Phys 9:3864–3871
Curtiss LA, Raghavachari K, Trucks GW, Pople JA (1991) J Chem Phys 94:7221–7230
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PM W, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, Revision B.03. Gaussian Inc, Pittsburgh PA
Johnston HS (1966) Gas-Phase Reaction Rate Theory. Ronald, New York
Laidler KJ (1969) Theories of Chemical Reaction Rates. McGraw-Hill, New York
Burk P, Koppel IA, Rummel A, Trumlar A (2000) J Phys Chem A 104:1602–1607
Mozurkevich M, Benson SW (1984) J Phys Chem 88:6429–6435
Chen Y, Rauk A, Tschuikow-Roux E (1991) J Phys Chem 95:9900–9908
Jodkowski JT, Rayez MT, Rayez JC, Bérces T, Dóbé S (1998) J Phys Chem A 102:9219–9229
Jodkowski JT, Rayez MT, Rayez JC, Bérces T, Dóbé S (1998) J Phys Chem A 102:9230–9243
Jodkowski JT, Rayez MT, Rayez JC, Bérces T, Dóbé S (1999) J Phys Chem A 103:3750–3765
Acknowledgments
This research was supported by Wroclaw Medical University under grant no. ST-263. The Wroclaw Center of Networking and Supercomputing is acknowledged for the generous allotment of computer time.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brudnik, K., Jodkowski, J.T., Sarzyński, D. et al. Mechanism of the gas-phase decomposition of trifluoro-, trichloro-, and tribromomethanols in the presence of hydrogen halides. J Mol Model 17, 2395–2409 (2011). https://doi.org/10.1007/s00894-011-0988-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-0988-5