Abstract
An all-electron scalar relativistic calculation was performed on Au n H2O (n = 1–13) clusters using density functional theory (DFT) with the generalized gradient approximation at PW91 level. The calculation results reveal that, after adsorption, the small gold cluster would like to bond with oxygen and the H2O molecule prefers to occupy the single fold coordination site. Reflecting the strong scalar relativistic effect, Au n geometries are distorted slightly but still maintain a planar structure. The Au–Au bond is strengthened and the H–O bond is weakened, as manifested by the shortening of the Au–Au bond-length and the lengthening of the H–O bond-length. The H–O–H bond angle becomes slightly larger. The enhancement of reactivity of the H2O molecule is obvious. The Au–O bond-lengths, adsorption energies, VIPs, HLGs, HOMO (LUMO) energy levels, charge transfers and the highest vibrational frequencies of the Au–O mode for Au n H2O clusters exhibit an obvious odd-even oscillation. The most favorable adsorption between small gold clusters and the H2O molecule takes place when the H2O molecule is adsorbed onto an even-numbered Au n cluster and becomes an Au n H2O cluster with an even number of valence electrons. The odd–even alteration of magnetic moments is observed in Au n H2O clusters and may serve as material with a tunable code capacity of “0” and “1” by adsorbing a H2O molecule onto an odd or even-numbered small gold cluster.

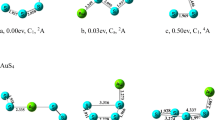
Lowest energy geometry for Au10H2O cluster. The average Au–Au, Au–O, and H–O bond-lengths (in Ångstrom) and the H–O–H bond angle are shown next to the cluster

Size dependence of vertical ionization potentials (VIP), HOMO–LUMO gaps (HLG), highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy level for Au n H2O clusters








Similar content being viewed by others
References
Meng S, Wang EG, Gao SW (2004) Phys Rev B 69:195404–195416
Li JB, Zhu SL, Li Y, Wang FH (2007) Phys Rev B 76:235433–235440
Cho JH, Kleinman L (2002) Phys Rev B 66:113306–113309
Materzanini G, Tantardini GF, Lindan PJD, Saalfrank P (2005) Phys Rev B 71:155414–155430
Blanco R, Orts JM (2008) Electrochim Acta 53:7796–7804
Henderson MA (2002) Surf Sci Rep 46:1–308
Taylor CD, Neurock M (2005) Curr Opin Solid State Mater Sci 9:49–65
Clay C, Hodgson A (2005) Curr Opin Solid State Mater Sci 9:11–18
Suzuki Y, Yamashita K (2010) Chem Phys Lett 486:48–52
Prestianni A, Martorana A, Labat F, Ciofini I, Adamo C (2009) J Mol Struct Theochem 903:34–40
Padilla-Campos L (2008) J Mol Struct Theochem 851:15–21
Assadollahzadeh B, Schwerdtfeger P (2009) J Chem Phys 131:064306–064316
Kang GJ, Chen ZX, Li Z, He X (2009) J Chem Phys 130:034701–034706
Li GP, Hamilton IP (2006) Chem Phys Lett 420:474–479
Chrétien S, Buratto SK, Metiu H (2007) Curr Opin Solid State Mater Sci 11:62–75
Meier DC, Goodman DW (2004) J Am Chem Soc 126:1892–1899
Bernhardt TM (2005) Int J Mass Spectrom 243:1–29
Haruta M, Tsubota S, Kobayashi T, Kageyama H, Genet MJ, Delmon B (1993) J Catal 144:175–192
Boccuzzi F, Chiorino A, Manzoli M, Haruta M (2001) J Catal 202:256–267
Mul G, Zwijnenburg A, van der Linden B, Makkee M, Moulijn JA (2001) J Catal 201:128–137
Jia JF, Haraki K, Kondo JN, Domen K, Tamaru K (2000) J Phys Chem B 104:11153–11156
Sárkány A, Révay Z (2003) Appl Catal A:Gen 243:347–355
Ju SP (2005) J Chem Phys 122:094718–094723
Chang CI, Lee WJ, Young TF, Ju SP, Chang CW, Chen HL, Chang JG (2008) J Chem Phys 128:154703–154712
Weng MH, Lee WJ, Ju SP, Chao CH, Hsieh NK, Chang JG, Chen HL (2008) J Chem Phys 128:174705–174713
Choudhary TV, Goodman DW (2002) Top Catal 21:1–12
Fernandez EM, Soler JM, Garzon LL, Balbas C (2004) Phys Rev B 70:165403–165416
Orita H, Itoh N, Inada Y (2004) Chem Phys Lett 384:271–276
Lee YS, McLean AD (1982) J Chem Phys 76:735–736
Datta SN, Ewig CS (1982) Chem Phys Lett 85:443–446
Hakkinen H, Landman U (2000) Phys Rev B 62:R2287–R2290
Myoung H, Ge M, Sahu BR, Tarakeswar P, Kim KS (2003) J Chem Phys 107:9994–10002
Fernandez EM, Soler JM, Garzon LL, Balbas C (2004) Phys Rev B 70:165403–165416
Mao HP, Wang HY, Ni Y, Xu GL (2004) Acta Phys Sin 53:1766–1771
Deka A, Deka RC (2008) J Mol Struct Theochem 870:83–93
Hakkinen H, Yoon B, Landman U, Li X, Zhai HJ, Wang LS (2003) J Phys Chem A 107:6168–6175
Feller D, Glendening ED, de Jong WA (1999) J Chem Phys 110:1475–1491
Schröder D, Schwarz H, Hrušák J, Pyykkö P (1998) Inorg Chem 37:624–632
(1993-1994) In: Lide DR (ed) CRC Handbook of chemistry and physics. Chemical Rubber Company, Boca Raton, pp74–75
Cao ZX, Wang YJ, Zhu J, Wu W, Zhang Q (2002) J Phys Chem B106:9649–9654
Poater A, Duran M, Jaque P, Toro-Labbe A, Sola M (2006) J Phys Chem B 110:6526–6536
Ding XL, Li ZY, Yang JL, Hou JG, Zhu QS (2004) J Chem Phys 121:2558–2562
Wu X, Senapati L, Nayak SK, Selloni A, Hajaligol M (2002) J Chem Phys 117:4010–4015
Ghebriel HW, Kshirsagar A (2007) J Chem Phys 126:244705–244713
Phala S, Klatt G, Steen EV (2004) Chem Phys Lett 395:33–37
Eberhart ME, Handley RC, Johnson KH (1984) Phys Rev B 29:1097–1100
Ding XL, Li ZY, Yang JL, Hou JG, Zhu QS (2004) J Chem Phys 120:9594–9601
Zhang M, He LM, Zhao LX, Feng XJ, Cao W, Luo Y (2009) J Mol Struct Theochem 911:65–69
Torres M, Fernández E, Balbás L (2006) Phys Rev B 71:155412–155418
Majumder C, Kandalam A, Jena P (2006) Phys Rev B 74:205437–205442
Janssens E, Tanaka H, Neukermans S, Silverans RE, Lievens P (2004) Phys Rev B69:085402–085410
Panas I, Siegbahn P, Walhgren U (1987) Chem Phys 112:325–337
Acknowledgment
This work is supported by the Nature Science Foundation of Chongqing city. No. CSTC - 2007BB4137.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kuang, Xj., Wang, Xq. & Liu, Gb. All-electron scalar relativistic calculation of water molecule adsorption onto small gold clusters. J Mol Model 17, 2005–2016 (2011). https://doi.org/10.1007/s00894-010-0910-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0910-6