Abstract
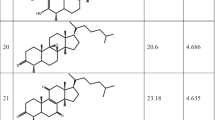
For the first time, a set of (43) natural sesquiterpene polyol esters isolated from the root bark of Celastrus angulatus Maxim and Euonymus japonicus Thunb were subjected to 3D-QSAR comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) studies, with the aim of proposing novel sesquiterpene-based compounds with optimal narcotic or insecticidal activities. The established 3D-QSAR models exhibit reasonable statistical quality and prediction capabilities, with internal cross-validated Q 2 values of ∼0.5 and external predicted R 2 values of >0.9, respectively. The relative contributions of the steric/electrostatic fields of the 3D-QSAR models show that the electronic effect governs the narcotic activities of the molecules, but the hybrid effect of the electrostatic and hydrophobic interactions is more influential in the insecticidal activities of the compounds. These findings may have valuable implications for the development of novel natural insecticides.

A 3D-QSAR study using CoMFA and CoMSIA methods was carried out on a series of 43 natural insecticidal/narcotic sesquiterpene polyol esters. The CoMSIA models showed excellent predictive capabilities.










Similar content being viewed by others
References
Grabley S, Thiericke R (1999) Adv Biochem Eng/Biotechnol 64:101–154
Cooper EL (2004) eCAM 1:215–217
Sparks TC, Crouse GD, Durst G (2001) Pest Manag Sci 57:896–905
Isman MB, Akhtar Y (2007) Plant natural products as a source for developing environmentally acceptable insecticides. In: Ishaaya I, Nauen R, Horowitz AR (eds) Insecticide design using advanced technologies. Springer, Berlin, pp 235–248
Gao JM, Wu WJ, Zhang JW (2007) Nat Prod Rep 24:1153–1189
Cortés-Selva F, Campillo M, Reyes CP, Jiménez IA, Castanys S, Bazzocchi IL, Pardo L, Gamarro F, Ravelo AG (2004) J Med Chem 47:576–587
Gonzalez AG, Jiminez IA, Ravelo AG, Coll J, Gonzalez JA, Lloria (1997) J Biochem Syst Ecol 25:513–519
Cheng CY, Huang PH (1999) Flora Reipublicae Popularis Sinicae 3(45). Science Press, Beijing, pp 7–128
Ji ZQ, Hu ZN, Liu GQ, Wu W (2004) J Acta Bot Boreali-Occidentalia Sin 24:748–753
Zhan JW, Wu WJ, Tian X (2004) Chin J Pesticide Sci 6:21–25
Wu W (1991) J Plant Prot 17:34–38
Spivey AC, Weston M, Woodhead S (2002) Chem Soc Rev 31:43–59
Cortes-Selva F, Jimenez IA, Munoz-Martinez F, Campillo M, Bazzocchi IL, Pardo L, Ravelo AG, Castanys S, Gamarro F (2005) Curr Pharm Des 11:3125–3139
Reyes CP, Muñoz-Martinez F, Torrecillas IR, Mendoza CR, Gamarro F, Bazzocchi IL, Núñez MJ, Pardo L, Castanys S, Campillo M, Jiménez IA (2007) J Med Chem 50:4808–4817
Zhang YL, Xu Y, Lin JF (1989) Acta Pharmacol Sin 24:568–578
Duan H, Takaishi Y, Momota H, Ohmoto Y, Taki T, Tori M, Takaoka S, Jia Y, Li D (2001) Tetrahedron 57:8413–8424
Kuo Y, King M, Chen C, Chen H, Chen C, Chen K, Lee KJ (1994) Nat Prod 57:263–269
González AG, San Andrés L, Ravelo AG, Luis JG, Jiménez IA, Domínguez XA (1989) J Nat Prod 52:1338–1341
Perez-Victoria JM, Tincusi BM, Jimenez IA, Bazzocchi IL, Gupta MP, Castanys S, Gamarro F, Ravelo AG (1999) J Med Chem 42:4388–4393
Cortes-Selva F, Munoz-Martinez F, Ilias A, Jimenez AI, Varadi A, Gamarro F, Castanys S (2005) Biochem Biophys Res Commun 329:502–507
Muñoz-Martinez F, Lu P, Cortes-Selva F, Perez-Victoria JM, Jimenez AI, Ravelo AG, Sharom FJ, Gamarro F, Castanys S (2004) Cancer Res 64:7130–7138
Ujita K, Takaishi Y, Tokuda H, Nishino H, Iwashima A, Fujita T (1993) Cancer Lett 68:129–133
Takaishi Y, Ujita K, Tokuda H, Nishino H, Iwashima A, Fujita T (1992) Cancer Lett 65:19–26
Wei SP, Wang MA, Zhang JW, Qian Y, Ji ZQ, Wu WJ (2009) Nat Prod Commun 4:461–466
Wei SP, Ji ZQ, Zhang JW (2009) Molecules 14:1396–1403
Wu WJ, Wang MA, Zhu JB, Zhou WM, Hu ZN, Ji ZQ (2001) J Nat Prod 64:364–367
Wu WJ, Tu YQ, Zhu JB (1992) J Nat Prod 55:1294–1298
Gonzalez AG, Gonzalez CM, Bazzochi IL, Ravelo AG, Luis JG, Dominguez XA (1987) Phytochemistry 26:2133–2135
Yamada K, Shizuri Y, Hirata Y (1978) Tetrahedron 34:1915–1920
Wu MJ, Zhao TZ, Shang Y (2004) J Chinese Chem Lett 15:41–42
Ji ZQ, Wu WJ, Yang H (2007) Nat Prod Res 21:334–342
Di Santo R, Fermeglia M, Ferrone M, Paneni MS, Costi R, Artico M, Roux A, Gabriele M, Tardif KD, Siddiqui A, Pricl S (2005) J Med Chem 48:6304–6314
Clark M, Cramer RD III, van Opdenbosch N (1989) J Comput Chem 10:982–1012
Nilsson J (1998) Multiway calibration in 3D QSAR. http://www.ub.rug.nl/eldoc/dis/science/j.nilsson
Klebe G, Abraham U, Mietzner T (1994) J Med Chem 37:4130–4146
Viswanadhan VN, Ghose AK, Revenkar GR, Robins R (1989) J Chem Inf Comput Sci 29:163–172
Klebe G (1994) J Mol Biol 237:212–235
Staahle L, Wold S (1987) J Chemometr 1:185–196
Li YF, Liu YL, Song ZQ (2006) Agrochemicals 45:148–154
Roy KK, Dixit A, Saxena AK (2008) J Mol Graph Model 27:197–208
Golbraikh A, Tropsha A (2002) J Mol Graph Model 20:269–276
Clark RD, Sprous DG, Leonard JM (2001) Validating models based on large dataset. In: Holtje HD, Sippl W (eds) Rational approaches to drug design (Proceedings of the 13th European Symposium on Quantitative Structure–Activity Relationships). Prous Science, Barcelona, pp 475–485
Acknowledgments
These projects were financed by the National Key S&T Research Foundation of China (2010CB126105) and the National Natural Science Foundation of China (30871663).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shao-peng Wei, Zhi-qin Ji and Hui-xiao Zhang have contributed equally.
Rights and permissions
About this article
Cite this article
Wei, Sp., Ji, Zq., Zhang, Hx. et al. Isolation, biological evaluation and 3D-QSAR studies of insecticidal/narcotic sesquiterpene polyol esters. J Mol Model 17, 681–693 (2011). https://doi.org/10.1007/s00894-010-0765-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0765-x