Abstract
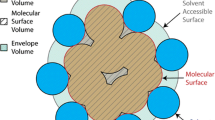
In this paper, we describe a Monte Carlo method for determining the volume of a molecule. A molecule is considered to consist of hard, overlapping spheres. The surface of the molecule is defined by rolling a probe sphere over the surface of the spheres. To determine the volume of the molecule, random points are placed in a three-dimensional box, which encloses the whole molecule. The volume of the molecule in relation to the volume of the box is estimated by calculating the ratio of the random points placed inside the molecule and the total number of random points that were placed. For computational efficiency, we use a grid-cell based neighbor list to determine whether a random point is placed inside the molecule or not. This method in combination with a graph-theoretical algorithm is used to detect internal cavities and surface clefts of molecules. Since cavities and clefts are potential water binding sites, we place water molecules in the cavities. The potential water positions can be used in molecular dynamics calculations as well as in other molecular calculations. We apply this method to several proteins and demonstrate the usefulness of the program. The described methods are all implemented in the program McVol, which is available free of charge from our website at http://www.bisb.uni-bayreuth.de/software.html.









Similar content being viewed by others
References
Kawabata T, Go N (2007) Detection of pockets on protein surfaces using small and large probe spheres to find putative ligand binding sites. Structure 68:516–529
Liang J, Edelsbrunner H, Woodward C (1998) Anatomy of protein pockets and cavities: measurement of binding site geometry and implications for ligand design. Prot Sci 7:1884–1897
Thornton JM, Todd AE, Milburn D, Borkakoti N, Orengo CA (2000) From structure to function: approaches and limitations. Nat Struct Biol 7:991–994
Kuntz ID, Blaney JM, Oatley SJ, Langridge R, Ferrin TE (1982) A geometric approach to macromolecule-ligand interactions. J Mol Biol 161:269–288
Delaney JS (1992) Finding and filling protein cavities using cellular logic operations. J Mol Graph 10:174
Warshel A (2002) Molecular dynamics simulations of biological reactions. Acc Chem Res 35:385–395
Till MS, Essigke T, Becker T, Ullmann GM (2008) Simulating the proton transfer in gramicidin a by a sequential dynamical Monte Carlo method. J Phys Chem, B 112:13401–13410
Bondar AN, Elstner M, Suhai S, Smith JC, Fischer S (2004) Mechanism of primary proton transfer in bacteriorhodopsin. Structure 12:1281–1288
Edelsbrunner H, Mucke EP (1990) Simulation of simplicity - a techique to cope with degenerate cases in geometric algorithms. ACM Trans Graph 9:66–104
Xie L, Bourne PE (2007) A robust and efficient algorithm for the shape description of protein structures and its application in predicting ligand binding sites. Bioinformatics 8
Hendlich M, Rippmann F, Barnickel G (1997) LIGSITE: automatic and efficient detection of potential small molecule-binding sites in proteins. J Mol Graph 15:359
Levitt DG, Banaszak LJ (1992) POCKET - A computer-graphics method for identifying and displaying protein cavities and their surrounding amino acids. J Mol Graph 10:229–234
Laskowski RA (1995) SURFNET - A program for visualizing molecular surfaces, cavities and intermolecular interactions. J Mol Graph 13:323
Allen MP, Tildesley DJ (1989) Computer simulation of liquids. Oxford University Press
Lee B, Richards FM (1971) Interpretation of protein structures - estimation of static accessibility. J Mol Biol 55:379
Eisenhaber F, Lijnzaad P, Argos P, Sander C, Scharf M (1995) The double cubic lattice method - efficient approaches to numerical-integration of surface-area and volume and to dot surface contouring of molecular assemblies. J Com Chem 16:273–284
Press WH, Teukolsky SA, Vetterling WT, Flannery BP (1992) Numerical recipes in C, 2nd edn. Cambridge University Press, Cambridge UK
Sedgewick (R) Algorithms in C++, part 5. Addison-Wesley, Boston
M. Masuya and J. Doi. Detection and Geometric Modeling of Molecular Surfaces and Cavities Using Digital Mathematical Morphological Operations. J Mol Graph, 13:331, 1995.
Peters KP, Fauck J, Frommel C (1996) The automatic search for ligand binding sites in proteins of known three-dimensional structure using only geometric criteria. J Mol Biol 256:201–213
Ruppert J, Welch W, Jain AN (1997) Automatic identification and representation of protein binding sites for molecular docking. Prot Sci 6:524–533
Brady GP, Stouten PFW (2000) Fast prediction and visualization of protein binding pockets with PASS. J Comput Aided Mol Design 14:383–401
Venkatachalam CM, Jiang X, Oldfield T, Waldman M (2003) Ligandfit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J Mol Graph 21:289–307
Laurie ATR, Jackson RM (2005) Q-SiteFinder: an energy-based method for the prediction of protein-ligand binding sites. Bioinformatics 21:1908–1916
Calimet N, Ullmann GM (2004) The influence of a transmembrane ph gradient on protonation probabilities of bacteriorhodopsin: the structural basis of the back-pressure effect. J Mol Bio 339(3):571–589
Koepke J, Krammer E-M, Klingen AR, Sebban P, Ullmann GM, Fritzsch G (2007) pH modulates the quinone position in the photosynthetic reaction center from rhodobacter sphaeroides in the neutral and charge separated states. J Mol Biol 371:396–409
Klingen AR, Palsdottir H, Hunte C, Ullmann GM (2007) Redox- linked protonation state changes in cytochrome bc1 identified by Poisson-Bolt zmann electrostatics calculations. Biochem Biophys Acta 1767:204–221
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminatha S, Karplus M (1983) CHARMM - A programm for macromolecular energy, minimization, and dynamics calculations. J Com Chem 4:187–217
Bondi A (1964) Van der Waals volumes and radii. J Phys Chem 68:441
Hiromoto T, Fujiwara S, Hosokawa K, Yamaguchi H (2006) Crystal structure of 3- hydroxybenzoate hydroxylase from comamonas testosteroni has a large tunnel for substrate and oxygen access to the active site. J Mol Biol 364:878–896
Borodich AI, Ullmann GM (2004) Internal hydration of protein cavities: studies on BPTI. Phys Chem 6:1906–1911
Takano K, Funahashi J, Yamagata Y, Fujii S, Yutani K (1997) Contribution of water molecules in the interior of a protein to the conformational stability. J Mol Biol 274:132–142
Otting G, Liepinsh E, Halle B, Frey U (1997) NMR identification of hydrophobic cavities with low water occupancies in protein structures using small gas molecules. Nat Struct Biol 4:396–404
Kodanadapani R, Suresh CG, Vijayan M (1990) Crystal-structure of low humidity tetragonal lysozyme at 2.1A resolution. J Biol Chem 265:16126–16131
Lanyi JK (2006) Proton transfers in the bacteriorhodopsin photocycle. Bioch et Biophys Acta - Bioener 1757:1012–1018
Heberle J (2000) Proton transfer reactions across bacteriorhodopsin and along the membrane. Bioch et Biophys Acta - Bioener 1458:135–147
Okamura MY, Paddock ML, Graige MS, Feher G (2000) Proton and electron transfer in bacterial reaction centers. Biochim Biophys Acta 1458:148–163
Stowell MHB, McPhillips TM, Rees DC, Soltis SM, Abresch E, Feher G (1997) Light-induced structural changes in photosynthetic reaction center: implications for mechanism of electron-proton transfer. Science 276:812–816
Paddock ML, Feher G, Okamura MY (2003) Proton transfer pathways and mechanism in bacterial reaction centers. FEBS Lett 555:45–50
Parkin S, Rupp B, Hope H (1996) Structure of bovine pancreatic trypsin inhibitor at 125K: definition of carboxyl-terminal residues Gly57 and Ala58. Acta Crystallogr, Sect.D 52:18–29
Jung A, Domratcheva T, Tarutina M, Wu Q, Ko WH, Shoeman RL, Gomelsky M, Gardner KH, Schlichting L (2005) Structure of a bacterial BLUF photoreceptor: insights into blue light-mediated signal transduction. Proc Natl Acad Sci USA 102:12350–12355
Brownlow S, Cabral JHM, Cooper R, Flower DR, Yewdall SJ, Polikarpov I, North ACT, Sawyer L (1997) Bovine Beta-lactoglobulin at 1.8 angstrom resolution - still an enigmatic lipocalin. Structure 5:481–495
Nogues I, Perez-Dorado I, Frago S, Bittel C, Mayhew SG, Gomez-Moreno C, Hermoso JA, Medina M, Cortez N, Carrillo N (2005) The Ferredoxin-NADP(H) reductase from rhodobacter capsulatus: molecular structure and catalytic mechanism. Biochemistry 44:11730–11740
Luecke H, Schobert B, Richter HT, Cartailler JP, Lanyi JK (1999) Structure of bacteriorhodopsin at 1.55 angstrom resolution. J Mol Biol 291:899–911
Retailleau P, Colloc’h N, Vivares D, Bonnete F, Castro B, El Hajji M, Mornon JP, Monard G, Prange T (2004) Complexed and ligand-free high-resolution structures of Urate Oxidase (Uox) from aspergillus flavus: a reassignment of the active-site binding. Acta Crystallogr, Sect.D 60:453–462
Andrade SLA, Dickmanns A, Ficner R, Einsle O (2005) Crystal structure of the archaeal ammonium transporter amt-1 from archaeoglobus fulgidus. Proc Natl Acad Sci USA 102:14994–14999
Fujimoto Z, Takase K, Doui N, Momma M, Matsumoto T, Mizuno H (1998) Crystal structure of a catalytic-site mutant alpha-amylase from bacillus subtilis complexed with maltopentaose. J Mol Biol 277:393–407
Brudler R, Hitomi K, Daiyasu H, Toh H, Kucho K, Ishiura M, Kanehisa M, Roberts VA, Todo T, Tainer JA, Getzoff ED (2003) Identification of a new cryptochrome class: structure, function, and evolution. Mol Cell 11:59–67
Wohlfahrt G, Witt S, Hendle J, Schomburg D, Kalisz HM, Hecht HJ (1999) 1.8 and 1.9 angstrom resolution structures of the penicillium amagasakiense and aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr, Sect.D 55:969–977
Hohenester E, Maurer P, Timpl R (1997) Crystal structure of a pair of follistatin-like and EF-hand calcium-binding domains in BM-40. EMBO J 16:3778–3786
Seiffert GB, Ullmann GM, Messerschmidt A, Schink B, Kroneck PMH, Einsle O (2007) Structure of the non-redox-active tungsten/[4Fe : 4S] enzyme acetylene hydratase. Proc Natl Acad Sci USA 104:3073–3077
Acknowledgements
This work was supported by the DFG grant UL 174/7-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Till, M.S., Ullmann, G.M. McVol - A program for calculating protein volumes and identifying cavities by a Monte Carlo algorithm. J Mol Model 16, 419–429 (2010). https://doi.org/10.1007/s00894-009-0541-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0541-y