Abstract
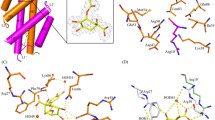
Bacillus anthracis has been employed as an agent of bioterrorism, with high mortality, despite anti-microbial treatment, which strongly indicates the need of new drugs to treat anthrax. Shikimate pathway is a seven step biosynthetic route which generates chorismic acid from phosphoenol pyruvate and erythrose-4-phosphate. Chorismic acid is the major branch point in the synthesis of aromatic amino acids, ubiquinone, and secondary metabolites. The shikimate pathway is essential for many pathological organisms, whereas it is absent in mammals. Therefore, these enzymes are potential targets for the development of nontoxic antimicrobial agents and herbicides and have been submitted to intensive structural studies. The forth enzyme of this pathway is responsible for the conversion of dehydroshikimate to shikimate in the presence of NADP. In order to pave the way for structural and functional efforts toward development of new antimicrobials we describe the molecular modeling of shikimate dehydrogenase from Bacillus anthracis complexed with the cofactor NADP. This study was able to identify the main residues of the NADP binding site responsible for ligand affinities. This structural study can be used in the design of more specific drugs against infectious diseases.









Similar content being viewed by others
References
Casadevall A (2008) Front Biosci 13:4009
Oliveira JS, Sousa EHS, Basso LA, Palaci M, Dietze R, Santos DS et al (2004) Chem Commun (Camb) 7:312 doi:10.1039/b313592f
Oliveira JS, Sousa EHS, Souza ON, Moreira IS, Santos DS, Basso LA (2006) Curr Pharm Des 12:2409 doi:10.2174/138161206777698927
Oliveira JS, Vasconcelos IB, Moreira IS, Santos DS, Basso LA (2007) Curr Drug Targets 8:399 doi:10.2174/138945007780058942
Dias MV, Vasconcelos IB, Prado AM, Fadel V, Basso LA, de Azevedo WF Jr et al (2007) Struct Biol 159:369 doi:10.1016/j.jsb.2007.04.009
Vasconcelos IB, Meyer E, Sales FAM, Moreira IS, Basso LA, Santos DS (2008) Anti-infecive Agents in Medicinal Chemistry 7:50
Tipparaju SK, Jovasawal S, Forrester S, Mulhearn DC, Pegan S, Johnson ME et al (2008) Bioorg Med Chem Lett 18(12):3565 doi:10.1016/j.bmcl.2008.05.004
Pereira JH, Vasconcelos JB, Oliveira JS, Caceres RA, de Azevedo WF Jr, Basso LA et al (2007) Curr Drug Targets 8:459 doi:10.2174/138945007780059013
Dias MV, Faím LM, Vasconcelos IB, de Oliveira JS, Basso LA, Santos DS et al (2007) Acta Crystallogr Sect F Struct Biol Cryst Commun 63:1 doi:10.1107/S1744309106046823
Silveira NJ, Uchoa HB, Pereira JH, Canduri F, Basso LA, Palma MS et al (2005) J Mol Model 11:160 doi:10.1007/s00894-005-0240-2
Pereira JH, de Oliveira JS, Canduri F, Dias MV, Palma MS, Basso LA et al (2004) Acta Crystallogr D Biol Crystallogr 60:2310 doi:10.1107/S090744490402517X
Segura-Cabrera A, Rodríguez-Pérez MA (2008) Bioorg Med Chem Lett 18(11):3152 doi:10.1016/j.bmcl.2008.05.003
de Azevedo WF Jr (2007) Curr Drug Targets 8(3):387 doi:10.2174/138945007780058960
Herrmann KM, Weaver LM (1999) Annu Rev Plant Physiol Plant Mol Biol 50:473–503 doi:10.1146/annurev.arplant.50.1.473
Steinrucken HC, Amrhein N (1984) Eur J Biochem 14:351–357 doi:10.1111/j.1432-1033.1984.tb08379.x
Schonbrunn E, Eschenburg S, Shuttleworth WA, Schloss JV, Amrhein N, Evans JN (2001) Proc Natl Acad Sci USA 98:1376–1380 doi:10.1073/pnas.98.4.1376
Benach J, Lee I, Edstrom W, Kuzin AP, Chiang Y, Acton TB et al (2003) J Biol Chem 278:19176–19182 doi:10.1074/jbc.M301348200
Shumilin I, Kretsinger R, Bauerle R (1999) Struct 7:865–875 doi:10.1016/S0969-2126(99)80109-9
Carpenter EP, Hawkins AR, Frost JW, Brown KA (1998) Nature 394:299–302 doi:10.1038/28431
Gourley DG, Shrive AK, Polikarpov I, Krell T, Coggins JR, Hawkins AR (1999) Nat Struct Biol 6:521–525 doi:10.1038/9287
Michel G, Roszak AW, Sauve V, Maclean J, Matte A, Coggins JR (2003) J Biol Chem 278:19463–19472 doi:10.1074/jbc.M300794200
Krell T, Coggins JR, Lapthorn AJ (1998) J Mol Biol 278:983–997 doi:10.1006/jmbi.1998.1755
Stallings WC, Abdel-Maguid SS, Lim LW, Shie HS, Dayringer HE, Leimgruber NK (1991) Proc Natl Acad Sci USA 88:5046–5050 doi:10.1073/pnas.88.11.5046
Maclean J, Ali S (2003) Structure 11:1499–1511 doi:10.1016/j.str.2003.11.005
Bentley R (1990) Crit Rev Biochem Mol Biol 25(5):307–284 doi:10.3109/10409239009090615
Roberts F, Roberts CW, Johnson JJ, Kyle DE, Krell T, Coggins C et al (1998) Nature 393:801–805 doi:10.1038/30718
Roberts CW, Roberts F, Lyons RE, Kirisits MJ, Mui EJ, Finnerty J et al (2002) Infect Dis 185:25–36 doi:10.1086/338004
Herrmann KM (1995) Plant Cell 7:907–919
Murzin AG, Brenner SE, Hubbard T, Chothia C (1995) J Mol Biol 247:536–540
Baillie AC, Corbett JR, Dowsett JR, McCloskey P (1972) Biochem J 126:21
Deka RK, Anton IA, Dunbar B, Coggins JR (1994) FEBS Lett 349:397–402 doi:10.1016/0014-5793(94)00710-1
Bagautdinov B, Kunishima N (2007) J Mol Biol 373:424–438 doi:10.1016/j.jmb.2007.08.017
Kroemer RT, Doughty SW, Robinson AJ, Richards WG (1996) Protein Eng 9(6):493–498 doi:10.1093/protein/9.6.493
Berman HM, Westbrook J, Feng Z, Gilliard G, Bhat TN, Weissig H et al (2000) Nucleic Acids Res 28:235–242 doi:10.1093/nar/28.1.235
Fernandes CL, Breda A, Santos DS, Basso LA, Souza ON (2007) Comput Biol Med 37:149–158 doi:10.1016/j.compbiomed.2006.01.001
Gasteiger E, Jung E, Bairoch A (2001) Curr Issues Mol Biol 3:47
Bairoch A, Apweiler R (2000) Nucleic Acids Res 28:45 doi:10.1093/nar/28.1.45
Bairoch A, Apweiler RJ (1997) Mol Med 75:312
Uchoa HB, Jorge GE, da Silveira NJF, Camera JC Jr, Canduri A, de Azevedo WF (2004) Biochem Biophys Res Commun 325:1481–1486 doi:10.1016/j.bbrc.2004.10.192
Sali A, Blundell TL (1993) J Mol Biol 234:779–815 doi:10.1006/jmbi.1993.1626
Arcuri HA, Canduri F, Pereira JH, da Silveira NJF, Camara JC Jr, de Oliveira JS et al (1994) Biochem Biophys Res Commun 320:979–991 doi:10.1016/j.bbrc.2004.05.220
Dias MVB, Ely F, Palma MS, De Azevedo WF Jr, Basso LA, Santos DS (2007) Curr Drug Targets 8:48–55 doi:10.2174/138945007780058924
Thompson JD, Higgins DG, Gibson TJ (1994) Nucleic Acids Res 22:4673–4680 doi:10.1093/nar/22.22.4673
Marques MR, Pereira JH, Oliveira JS, Basso LA, Santos DS, De Azevedo WF Jr et al (2007) Curr Drug Targets 8:56–68 doi:10.2174/138945007780058951
Caceres RA, Timmers LFS, Dias R, Basso LA, Santos DS, de Azevedo WF (2008) Bioorg Med Chem 16(9):4984–4993 doi:10.1016/j.bmc.2008.03.044
De Azevedo WF, Mueller-Dieckmann JH, Schulze-Gahmen U, Worland PJ, Sausville E, Kim SH (1996) Proc Natl Acad Sci USA 93:2735–2740 doi:10.1073/pnas.93.7.2735
Wang R, Lai L, Wang S (2002) J Comput Aided J Mol Des 16:11 doi:10.1023/A:1016357811882
Caceres RA, Macedo Timmers LF, Vivan AL, Schneider CZ, Basso LA, De Azevedo WF et al (2008) J Mol Model 14(5):427–434 doi:10.1007/s00894-008-0291-2
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) J Appl Cryst 26:283–291 doi:10.1107/S0021889892009944
Wallace AC, Laskowski RA, Thornton JM (1995) Protein Eng 8:127–134 doi:10.1093/protein/8.2.127
Gasteiger E, Gattiker A, Hoogland C, Ivany I, Appel RD, Bairoch A (2003) Nucleic Acids Res 31:3784–3788 doi:10.1093/nar/gkg563
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph 14:33–38 doi:10.1016/0263-7855(96)00018-5
Delano WL, Lam JW (2005) Abstr Pap Am Chem Soc 230:1371–1372
Carugo O, Argos P (1997) Proteins 28:10–28 doi:10.1002/(SICI)1097-0134(199705)28:1<10::AID-PROT2>3.0.CO;2-N
Vogan E (2003) Structure 11:902–903 doi:10.1016/S0969-2126(03)00165-5
Arcuri HA, Borges JC, Fonseca IO, Pereira JH, Ruggiero-Neto J, Basso LA et al (2008) Proteins 72(2):720–730 doi:10.1002/prot.21953
Gan J, Wu Y, Prabakaram P, Gu Y, Li Y, Andrykovich M et al (2007) Biochemistry 46:9513–9522 doi:10.1021/bi602601e
Lim S, Schroder I, Monbouquette HG (2004) FEMS Microbiol Lett 238:101–106
Zhang X, Zhang S, Hao F, Lai X, Yu H, Huang Y et al (2005) Biochem Mol Biol 38:624–631
Han C, Wang L, Yu K, Chen L, Hu L, Chen K et al (2006) FEBS J 273:4682–4692 doi:10.1111/j.1742-4658.2006.05469.x
Collaborative Computation Project, Number 4 (1994) Acta Crystallogr D Biol Crystallogr 50:760 doi:10.1107/S0907444994003112
Brändén CI (1980) Q Rev Biophys 13:317–338
Lesk AM (1995) Curr Opin Struct Biol 5:775–783 doi:10.1016/0959-440X(95)80010-7
Canduri F, Teodoro LGVL, Lorenzi CCB, Hial V, Gomes RAS, Ruggiero Neto J et al (2001) Acta Crystallogr 57:1560–1570
De Azevedo WF Jr, Mueller-Dieckmann HJ, Schulze-Gahmen U, Worland PJ, Sausville E, Kim SH (1996) Proc Natl Acad Sci USA 93(7):2735–2740 doi:10.1073/pnas.93.7.2735
De Azevedo WF Jr, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH (1997) Eur J Biochem 243:518–526 doi:10.1111/j.1432-1033.1997.0518a.x
De Azevedo WF Jr, Canduri F, Fadel V, Teodoro LGVL, Hial V, Gomes RAS (2001) Biochem Biophys Res Commun 287(1):277–281
De Azevedo WF Jr, Canduri F, da Silveira NJF (2002) Biochem Biophys Res Commun 293(1):566–571 doi:10.1016/S0006-291X(02)00266-8
Kim SH, Schulze-Gahmen U, Brandsen J, de Azevedo WF Jr (1996) Prog Cell Cycle Res 2:137–145
Acknowledgments
This work was supported by grants from CNPq, CAPES and Instituto do Milênio (CNPq-MCT). WFA is senior researcher of CNPq (Conselho Nacional de Pesquisas, Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barcellos, G.B., Caceres, R.A. & de Azevedo, W.F. Structural studies of shikimate dehydrogenase from Bacillus anthracis complexed with cofactor NADP. J Mol Model 15, 147–155 (2009). https://doi.org/10.1007/s00894-008-0403-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-008-0403-z