Abstract
A comprehensive structural analysis of interactions involving amide NH and C=O groups in protein–ligand complexes has been performed based on 3,275 published crystal structures (resolution≤2.5 Å). Most of the amide C=O and NH groups at the protein–ligand interface are highly buried within the binding site and involved in H-bonds with corresponding counter-groups. Small percentages of C=O and NH groups are solvated or embedded in hydrophobic environments. In particular, C=O groups show a higher propensity to be solvated or embedded in a hydrophobic environment than NH groups do. A small percentage of carbonyl groups is involved in weak hydrogen bonds with CH. Cases of dipolar interactions, involving carbonyl oxygen and electrophilic carbon atoms, such as amide, amidinium, guanidium groups, are also identified. A higher percentage of NH are in contact with aromatic carbons, interacting either through hydrogen bonds (preferably with the NH group pointing towards a ring carbon atom) or through stacking between amide plane and ring plane. Comprehensive studies such as the present one are thought to be important for future improvements in the molecular design area, in particular for the development of new scoring functions.

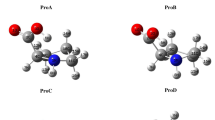
Example of dipolar interactions between carbonyl oxygen and amide carbons. a Dipolar (O carbonyl and C amide) interaction within an interaction network (red line H-bonds, blue line dipolar interaction). Protein: PDB 1a4h, [28] N terminal domain of the yeast HSP90 chaperone in complex with geldanamycin; b Dipolar interaction not in an interaction network (red line H-bonds, blue line dipolar interaction). Protein: PDB 1ezq, [29] Factor Xa









Similar content being viewed by others
References
Derewenda ZS, Lee L, Derewenda U (1995) J Mol Biol 252:248–262
Fabiola GF (1997) Acta Crystallogr D Biol Crystallogr 53:316–320
Klaholz B, Moras D (2002) Structure (Camb) 10:1197–1204
Pierce AC, Sandretto KL, Bemis GW (2002) Proteins 49:567–576
Manikandan K, Ramakumar S (2004) Proteins 56:768–781
Sarkhel S, Desiraju GR (2004) Proteins 54:247–259
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, Oxford
Steiner T (2002) Angew Chem Int Ed Engl 41:49–76
Steiner T, Koellner G (2001) J Mol Biol 305:535–557
Steiner T (2002) Biophys Chem 95:195–201
Steiner T, Schreurs AM, Kanters JA, Kroon J (1998) Acta Crystallogr D Biol Crystallogr 54 (Pt 1):25–31
Paulini R, Muller K, Diederich F (2005) Angew Chem Int Ed Engl (in press)
Allen FH (2002) Acta Crystallogr B 58:380–388
Hendlich M, Bergner A, Gunther J, Klebe G (2003) J Mol Biol 326:607–620
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) Nucleic Acids Res 28:235–242
Bergner A, Gunther J, Hendlich M, Klebe G, Verdonk M (2001) Biopolymers 61:99–110
OEChem Toolkit (2004) 1.3.2 OpenEye Scientific Software, Inc. (http://www.eyesopen.com)
James CA, Weininger D, Delany J (2001) Daylight Theory Manual. Daylight Chemical Information System, Inc, Los Altos
Stahl M, Bur D, Schneider G (1999) J Comput Chem 20:336–347
Mitchell JB, Nandi CL, McDonald IK, Thornton JM, Price SL (1994) J Mol Biol 239:315–331
Scheiner S, Kar T, Pattanayak J (2002) J Am Chem Soc 124:13257–13264
Thomas A, Meurisse R, Charloteaux B, Brasseur R (2002) Proteins 48:628–634
Toth G, Watts CR, Murphy RF, Lovas S (2001) Proteins 43:373–381
Weiss MS, Brandl M, Suhnel J, Pal D, Hilgenfeld R (2001) Trends Biochem Sci 26:521–523
Worth GA, Wade RC (1995) J Phys Chem-Us 99:17473–17482
Malone JF, Murray CM, Charlton MH, Docherty R, Lavery AJ (1997) J Chem Soc Faraday T 93:3429–3436
Sampson NS, Vrielink A (2003) Acc Chem Res 36:713–722
Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH (1997) Cell 90:65–75
Maignan S, Guilloteau JP, Pouzieux S, Choi-Sledeski YM, Becker MR, Klein SI, Ewing WR, Pauls HW, Spada AP, Mikol V (2000) J Med Chem 43:3226–3232
Dullweber F, Stubbs MT, Musil D, Sturzebecher J, Klebe G (2001) J Mol Biol 313:593–614
Krishnan R, Mochalkin I, Arni R, Tulinsky A (2000) Acta Crystallogr D Biol Crystallogr 56 (Pt 3):294–303
Peterson PE, Smith TJ (1999) Structure Fold Des 7:769–782
Ringhofer S, Kallen J, Dutzler R, Billich A, Visser AJ, Scholz D, Steinhauser O, Schreiber H, Auer M, Kungl AJ (1999) J Mol Biol 286:1147–1159
Maryanoff BE, Qiu X, Padmanabhan KP, Tulinsky A, Almond Jr HR, Andrade-Gordon P, Greco MN, Kauffman JA, Nicolaou KC, Liu A, Bungs PH, Fusetani N (1993) Proc Natl Acad Sci USA 90:8048–8052
Acknowledgements
The authors thank Prof. Klaus Mueller for stimulating discussions about non-bonded interactions, Dr. Harald Mauser and Dr. Olivier Roche for their suggestions and all colleagues at Roche Basel for a supportive research atmosphere.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cotesta, S., Stahl, M. The environment of amide groups in protein–ligand complexes: H-bonds and beyond. J Mol Model 12, 436–444 (2006). https://doi.org/10.1007/s00894-005-0067-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-005-0067-x