Abstract
Dirithromycin is a macrolide antibiotic derived from erythromycin A. Dirithromycin is synthesized by the condensation of 9(S)-erythromycylamine with 2-(2-methoxyethoxy)-acetaldehyde. To gain insight into the synthesis, the condensation mechanism has been analyzed computationally by the AM1 method in the gas phase. First, the formation of the Schiff bases of dirithromycin and epidirithromycin from 9(S)-erythromycylamine and 2-(2-methoxyethoxy)-acetaldehyde were modeled. Then, the tautomerization of the Schiff bases to dirithromycin and epidirithromycin were considered. Finally, the epimerization of the Schiff base of epidirithromycin to the Schiff base of dirithromycin was investigated. Our results show that, even though carbinolamine forms faster for epidirithromycin than the corresponding structure for dirithromycin, dirithromycin is the major product of the synthesis.
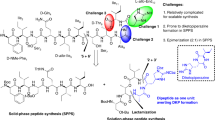
Figure Synthesis of dirithromycin








Similar content being viewed by others
References
Lingerfelt B, Champney WS (1999) J Pharm Biomed Anal 20:459–469
McGuire JM, Bunch PL, Anderson RC, Boaz HE, Flynn EH, Powell EH, Smith JW (1952) Antibiot Chemother 2:281–283
Corcoran JW (1984) In: Omura S (ed) Macrolide antibiotics: chemistry, biology and practice. Academic Press, Orlando, p 231
Clark RF, Ma Z, Wang S, Griesgraber G, Tufano M, Yong H, Li L, Zhang X, Nilius AM, Chu DTW, Or YS (2000) Bioorg Med Chem Lett 10:815–819
Sigal MV, Wiley PF, Gerzon K, Flynn EH, Quarck UC, Weaver O (1956) J Am Chem Soc 78:388–395
Massey EH, Kitchell BS, Martin LD, Gerzon K (1974) J Med Chem 17:105–107
Kirst HA, Wind JA, Leeds JP, Willard KE, Debono M, Bonjouklian R, Greene JM (1990) J Med Chem 33:3086–3094
Gill JM, Johnson R (1994) Tetrahedron 50:3857–3868
Luger P, Maier R (1979) J Crystallogr Mol Struct 9:329–338
Firl J, Prox A, Luger P, Maier R, Woitun E, Daneck K (1990) J Antibiot 43:1271–1277
Duran D, Aviyente V, Baysal C (2002) J Chem Soc Perkin Trans II 670–675
SPARTAN Version 5.1.3 (1998) Wavefunction Inc. 18401 Von Karman Ave. #370 Irvine, CA 92715
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratman RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu C, Liashenko A, Piskorz P, Komaromi, I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Gonzales C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Revision A.1. Gaussian, Pittsburgh, Pa.
Becke AD (1993) J Chem Phys 98:1372–1377
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Ditchfield R, Hehre WJ, Pople JA (1971) J Chem Phys 54:724–728
Hehre WJ, Ditchfield R, Pople JA (1972) J Chem Phys 56:2257–2261
Hariharan PC, Pople JA (1973) Theor Chim Acta 28:213–222
Barone V, Cossi M (1998) J Phys Chem A 102:1995–2001
Barone V, Cossi M, Tomasi J (1998) J Comput Chem 19:404–417
Gonzalez C, Schlegel HB (1989) J Phys Chem 90:2154–2161
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527
Hall NE, Smith BJ (1998) J Phys Chem A 102:4930–4938
Neuvonen K, Fulop F, Neuvonen H, Koch A, Kleinpeter E, Pihlaja K (2001) J Org Chem 66:4132–4140
Bahmanyar S, Houk KN (2001) J Am Chem Soc 123:11273–11283
Duran D, Aviyente V, Baysal C (2003) J Comput-Aided Mol Des (accepted for publication, 2004)
Acknowledgements
The authors would like to thank Boğaziçi Araştırma Fonu project 01M101 and TUBITAK (The Scientific and Technical Research Council of Turkey) Münir Birsel Foundation for financial support. The authors would also like to thank Dr. Nurcan Tüzün, Aliment Özen and Cem Öztürk for their efforts to support this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duran, D., Aviyente, V. & Baysal, C. A computational approach to the synthesis of dirithromycin. J Mol Model 10, 94–101 (2004). https://doi.org/10.1007/s00894-003-0172-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-003-0172-7