Abstract.
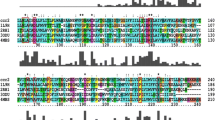
CCR2b, a chemokine receptor for MCP-1, -2, -3, -4, plays an important role in a variety of diseases involving infection, inflammation, and/or injury, as well as being a coreceptor for HIV-1 infection. Two models of human CCR2b (hCCR2b) were generated by homology modeling and 1 ns restrained molecular dynamics (MD) simulation. In one only C113–C190 forms a disulfide bond (SS model); in another the potential C32–C277 disulfide bond was formed (2SS model). Analysis of the structures and averaged displacements of Cα atoms of the N-terminal residues shows that the main differences between the SS and 2SS models lie in a region D25YDYGAPCHKFD36; in the extracellular part of the 2SS model the accessible surfaces of N12, F23, Y26, Y28 and F35 are obviously raised and a more stable H-bond net is formed. The potential energy of the 2SS–water assembly finally fluctuated around –43,020 kJ mol–1, which is about 302 kJ mol–1 lower than that of the SS–water assembly. All these results suggest that the 2SS model is more favorable. The CCR2b genes of 17 primates were sequenced and four CCR2b models for primates Ateles paniscus (A. pan), Hylobates leucogyneus (H. leu), Papio cynocephalus (P. cyn) and Trachypithecus francoist (T. fra) were generated based on the 2SS model. A comparison of hCCR2b with primate CCR2b also supports the importance of the region D25YDYGAPCHKFD36. Electrostatic potential maps of human and primate CCR2b all display the dipolar characteristics of CCR2b with the negative pole located in the extracellular part and a strong positive pole in the cytoplasmic part. Based on the CCR2b model, we suggest that the main functional residues fall in the D25YDYGAPCHKFD36 region, and the negative electrostatic feature is a non-specific, but necessary, factor for ligands or gp120/CD4 binding.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 5 April 2002 / Accepted: 21 May 2002 / Published online: 3 July 2002
Rights and permissions
About this article
Cite this article
Shi, XF., Liu, S., Xiangyu, J. et al. Structural analysis of human CCR2b and primate CCR2b by molecular modeling and molecular dynamics simulation. J Mol Model 8, 217–222 (2002). https://doi.org/10.1007/s00894-002-0089-6
Issue Date:
DOI: https://doi.org/10.1007/s00894-002-0089-6