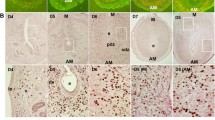
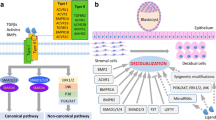
Abstract
Successful pregnancy requires coordination of embryo development, decidualization of endometrium, and placenta formation. Decidualization denotes the transformation of endometrial stromal cells into specialized secretory cells, a process further characterized with influx of specialized immune cells into stroma, predominantly uterine natural killer cells and macrophages, and vascular remodeling. This differentiation process depends on the convergence of the cyclic adenosine monophosphate and progesterone signaling pathways. The decidual process is indispensable for the formation of a functional feto-maternal interface as it controls tissue homeostasis during endovascular trophoblast invasion and bestows tissue resistance to environmental stress signals, including protection against oxidative cell death. FOXO proteins have emerged as key mediators of cell fate because of their ability to regulate either pro-apoptotic genes or genes involved in differentiation, cell cycle arrest, oxidative defenses, and DNA repair. In the endometrium, FOXO1 is of particular importance as a critical regulator of progesterone-dependent differentiation, menstrual shedding, and protection of the feto-maternal against oxidative damage during pregnancy.

Similar content being viewed by others
References
Simon C, Martin JC, Pellicer A (2000) Paracrine regulators of implantation. Baillieres Beat Pract Res Clin Obstet Gynaecol 14:815–826
Strowitzki T, Germeyer A, Popvici R, von Wolff M (2006) The human endometrium as a fertility-determining factor. Hum Reprod Update 12:617–630
Bergh PA, Navot D (1992) The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril 58:537–542
Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM (2010) Invasiveness of endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum Reprod 25:862–873
Gellersen B, Brosens IA, Brosens JJ (2007) Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453
Kajihara T, Jones M, Fusi L, Takano M, Feroze-Zaidi F, Pirianov G, Mehmet H, Ishihara O, Higham JM, Lam EW, Brosens JJ (2006) Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol Endocrinol 20:2444–2455
Brosens JJ, Pijnenborg R, Brosens IA (2002) The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 187:1416–1423
Gellersen B, Brosens J (2003) Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 178:357–372
Maruyama T, Yoshimura Y (2008) Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J 55:795–810
Brosens JJ, Hayashi N, White JO (1999) Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140:4809–4820
Brosens J, Brosens I (1998) Adenomyosis. In: Cameron I, Fraser I, Smith S (eds) Clinical disorders of the menstrual cycle and endometrium. Oxford University Press, New York, pp 297–312
Bulun SE, Noble LS, Takayama K, Michael MD, Agarwal V, Fisher C, Zhao Y, Hinshelwood MM, Ito Y, Simpson ER (1997) Endocrine disorders associated with inappropriately high aromatase expression. J Steroid Biochem Mol Biol 61:133–139
Daly DC, Maslar IA, Rosenberg SM, Tohan N, Riddick DH (1981) Prolactin production by luteal phase defect endometrium. Am J Obstet Gynecol 140:587–591
De Ziegler D, Fanchin R, de Moustier B, Bulletti C (1998) The hormonal control of endometrial receptivity: estrogen (E2) and progesterone. J Reprod Immunol 39:149–166
Telgmann R, Maronde E, Tasken K, Gellersen B (1997) Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology 138:929–937
Shalhegg BS, Tasken K (2000) Specificity in the cAMP/PKA signaling pathway, differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci 5:D678–D693
Mayr B, Montminy M (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2:599–609
Tseng L, Gao JG, Chen R, Zhu HH, Mazella J, Powell DR (1992) Effect of progestin, antiprogestin, and relaxin on the accumulation of prolactin and insulin-like growth factor-binding protein-1 messenger ribonucleic acid in human endometrial stromal cells. Biol Reprod 47:441–450
Tang B, Gurpide E (1993) Direct effect of gonadotropins on decidualization of human endometrial stromal cells. J Steroid Biochem Mol Biol 47:115–121
Frank GR, Brar AK, Cedars MI, Handwerger S (1994) Prostaglandin E2 enhances human endometrial stromal cell differentiation. Endocrinology 134:258–263
Ferrari A, Petraglia F, Gurpide E (1995) Corticotropin releasing factor decidualized human endometrial stromal cells in vitro. Interaction with progestin. J Steroid Biochem Mol Biol 54:251–255
Banerjee P, Sapru K, Strakova Z, Fazleabas AT (2009) Chorionic gonadotropin regulates prostaglandin E synthase via a phosphatidylinositol 3-kinase-extracellular regulatory kinase pathway in a human endometrial epithelial cell line: implications for endometrial responses for embryo implantation. Endocrinology 150:4326–4337
Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A, Fernandez de Mattos S, Lam EW, Brosens JJ (2006) Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol 20:35–44
Christian M, Zhang X, Schneider-Merck T, Unterman TG, Gellersen B, White JO, Brosens JJ (2002) Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein β in differentiating human endometrial stromal cells. J Bio Chem 277:20825–20832
Christian M, Pohnke Y, Kempf R, Gellersen B, Brosens JJ (2002) Functional association of PR and CCAAT/enhancer-binding protein β isoforms: promoter-dependent cooperation between PR-B and liver-enriched inhibitor protein, or liver-enriched activity protein and PR-A in human endometrial stromal cells. Mol Endocrinol 16:141–154
Mak IYH, Brosens JJ, Christian M, Hills FA, Chamley L, Regan L, White JO (2002) Regulated expression of signal transducer and activator of transcription, Stat5, and its enhancement of PRL expression in human endometrial stromal cells in vitro. J Clin Endocrinol Metab 87:2581–2588
Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H (1989) The homeotic gene forkhead encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell 57:645–658
Weigel D, Jackel H (1990) The fork head domain: a novel DNA binding motif of eukaryotic transcription factor. Cell 63:455–456
Calnan DR, Brunet A (2008) The FoxO code. Oncogene 27:2276–2288
Burgering BM (2008) A brief introduction to FOXOlogy. Oncogene 27:2258–2262
Dijker PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L, Coffer PJ (2000) Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27KIP1. Mol Cell Biol 20:9138–9148
Medema RH, Kops GJ, Bos JL, Burgering BM (2000) AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404:782–787
Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ (2000) Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-1. Curr Biol 10:1201–1204
Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW (2003) FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem 278:49795–49805
Modur V, Nagarajan R, Evers BM, Milbrandt J (2002) FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem 277:47928–47937
Suhara T, Kim HS, Kirshenbaum LA, Walsh K (2002) Suppression of Akt signaling induces Fas ligand expression: involvement of caspase and Jun kinase activation on Akt-mediated Fas ligand regulation. Mol Cell Biol 22:680–691
Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM (1999) Direct control of the forkhead transcription factor AFX by protein kinase B. Nature 398:630–634
Nemoto S, Finkel T (2002) Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 303:2011–2015
Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23:4802–4812
Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ Jr, DiStefano PS, Chiang LW, Greenberg ME (2002) DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296:530–534
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer OL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96:857–868
Burgering BM, Kops GJ (2002) Cell cycle and death control: long live forkheads. Trends Biochem Sci 27:352–360
Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME (2001) Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21:952–965
Woods YL, Rena G, Morrice N, Barthel A, Becker W, Guo S, Unterman TG, Cohen P (2001) The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem J 355:597–607
Rena G, Woods YL, Prescott AR, Peggie M, Unterman TG, Williams MR, Cohen P (2002) Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. EMBO J 21:2263–2271
Feroze-Zaidi F, Fusi L, Takano M, Higham J, Salker MS, Goto T, Edassery S, Klingel K, Boini KM, Palmada M, Kamps R, Groothuis PG, Lam EW, Smith SK, Lang F, Sharkey AM, Brosens JJ (2007) Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology 148:5020–5029
Vogt PK, Jiang H, Aoki M (2005) Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle 4:908–913
Wang MC, Bohmann D, Jasper H (2005) JNL extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121:115–125
Daitoku H, Hatta M, Matsuzai H, Arayani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A (2004) Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA 101:10042–10047
Matto MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L (2004) Mammalian SIRT1 represses forkhead transcription factors. Cell 116:551–563
Van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM (2004) FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2 (SIRT1). J Biol Chem 279:28873–28879
Meiese K, Chong ZZ, Shang YC, Hou J (2009) A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev 29:395–418
Kajihara T, Uchino S, Suzuki M, Itakura A, Brosens JJ, Ishihara O (2009) Increased ovarian follicle atresia in obese Zucker rats is associated with enhanced expression of the forkhead transcription factor FOXO1. Med Mol Morphol 42:216–221
Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG (2003) Regulation of insulin-like growth factor binding protein-1 activity by FKHR and HOXA10 in primate endometrial cells. Biol Reprod 68:24–30
Kim JJ, Buzzio OL, Li S, Lu Z (2005) Role of the FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod 73:833–839
Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M, Dina RE, Ishihara O, Takeda S, Lam EW, Bamberger AM, Ghaem-Maghami S, Brosens JJ (2008) Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene 27:9–19
Lam EW, Shah K, Brosens JJ (2012) The diversity of sex steroid action: the role of micro-RNAs and FOXO transcription factors in cycling endometrium and cancer. J Endocrinol 212:13–25
Kyo S, Sakaguchi J, Kiyono T, Shimizu Y, Maida Y, Mizumoto Y, Mori N, Nakamura M, Takakura M, Miyake K, Sakamoto M, Inoue M (2011) Forkhead transcription factor FOXO1 is a direct target of progestin to inhibit endometrial epithelial cell growth. Clin Cancer Res 17:525–537
Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ (2008) The regulation and function of the forkhead transcription factor, forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology 149:1942–1950
Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW, Unterman TG, Brosens JJ, Kim JJ (2007) Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 21:2334–2349
Tang M, Naidu D, Hearing P, Handwerger S, Tabibzadeh S (2010) LEFTY, a member of the transforming growth factor-beta superfamily, inhibits uterine stromal cell differentiation: a novel autocrine role. Endocrinology 151:1320–1330
Lynch VJ, Leclerc RD, May G, Wagner GP (2011) Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat Genet 43:1154–1159
Lynch VJ, Brayer K, Gellersen B, Wagner GP (2009) HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS ONE 4:e6845
Al-Sabbagh M, Fusi L, Higham J, Lee Y, Lei K, Hanyaloglu AC, Lam EW, Christian M, Brosens JJ (2011) NADPH oxidase-derived reactive oxygen species mediate decidualization of human endometrial stromal cells in response to cyclic AMP signaling. Endocrinology 152:730–740
Lynch VJ, May G, Wagner GP (2011) Regulatory evolution through divergence of a phosphoswitch in the transcription factor CEBPB. Nature 480:383–386
Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, Brosens IA (2009) A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol 200:615.e1–615.e6
Emera D, Romero R, Wagner G (2012) The evolution of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness. Bioessays 34:26–35
Burton GJ, Jauniaux E, Watson AL (1999) Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol 181:718–724
Burton GJ, Jauniaux E (2004) Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig 11:342–352
Brosens I, Robertson WB, Dixon HG (1967) The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol 93:569–579
Jauniaux E, Watoson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ (2000) Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 157:2111–2122
Lynch RE, Fridovich I (1978) Effects of superoxide on the erythrocyte membrane. J Biol Chem 25:1838–1845
Gellersen B, Brosens IA, Brosens JJ (2007) Decidualization of the human endometrium: mechanism, functions, and clinical perspectives. Semin Reprod Med 25:445–453
Leitao B, Jones MC, Fusi L, Higham J, Lee Y, Takano M, Goto T, Christian M, Lam EW, Brosens JJ (2010) Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J 24:1541–1551
Myatt L, Cui X (2004) Oxidative stress in the placenta. Histochem Cell Biol 122:369–382
Redman CW, Sargent IL (2005) Latest advances in understanding preeclampsia. Science 308:1592–1594
Sugino N, Nakata M, Kashida S, Karube A, Takiguchi S, Kato H (2000) Decreased superoxide dismutase expression and increased concentrations of lipid peroxide and prostaglandin F(2a) in the decidua of failed pregnancy. Mol Hum Reprod 6:642–647
Conde-Agudelo A, Romero R, Kusanovic JP, Hassan SS (2011) Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol 204:503.e1–503.e12
Kajihara T, Uchino S, Suzuki M, Itakura A, Brosens JJ, Ishihara O (2011) Human chorionic gonadotropin confers resistance to oxidative-induced apoptosis in decidualizing human endometrial stromal cells. Fertil Steril 95:1302–1307
Tesarik J, Hazout A, Medoza C (2003) Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod Biomed Online 7:59–64
Afshar Y, Miele L, Fazleabas AT (2012) Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology (in press)
Ricci G, Giolo E, Simeone R (2010) Heparin’s ‘potential to improve pregnancy rates and outcomes’ is not evidence-based. Hum Reprod Update 16:225–227
Urman B, Ata B, Yakin K, Alatas C, Aksoy S, Mercan R, Balaban B (2009) Luteal phase empirical low molecular weight heparin administration in patients with failed ICSI embryo transfer cycles: a randomized open-labeled pilot trial. Hum Reprod 24:1640–1647
Yamamoto H, Fuyama S, Arai S, Sendo F (1985) Inhibition of mouse natural killer cytotoxicity by heparin. Cell Immunol 96:409–417
Johann S, Zoller C, Haas S, Blumel G, Lipp M, Forster R (1995) Sulfated polysaccharide anticoagulants suppress natural killer cell activity in vitro. Thromb Haemost 74:998–1002
Christopherson KW, Campbell JJ, Travers JB, Hromas RA (2002) Low-molecular-weight heparins inhibit CCL2l-induced T cell adhesion and migration. J Pharmacol Exp Ther 302:290–295
Manduteanu I, Voinea M, Capraru M, Dragomir E, Simionescu M (2002) A novel attribute of enoxaparin: inhibition of monocyte adhesion to endothelial cells by a mechanism involving cell adhesion molecules. Pharmacology 65:52–53
Wan JG, Mu JS, Zhu HS, Geng JG (2002) N-desulfated non-anticoagulant heparin inhibits leukocyte adhesion and transmigration in vitro and attenuates acute peritonitis and ischemia and reperfusion injury in vitro. Inflamm Res 51:435–443
Fritchley SJ, Kirby JA, Ali S (1992) The antagonism of interferon-gamma (INF-gamma) by heparin: examination of the blockade of class II MHC antigen and heat shock protein-70 expression. Clin Exp Med Biol 120:247–252
Hills FA, Abrahams VM, Gonzalez-Timon B, Franvis J, Clock B, Hinkson L, Rai R, Mor Regan L, Sullivan M, Lam EW, Brosens JJ (2006) Heparin prevents programmed cell death in human trophoblast. Mol Hum Reprod 12:237–243
Leitao BB, Jones MC, Brosens JJ (2011) The SUMO E3-ligase PIAS1 couples reactive oxygen species-dependent JNK activation to oxidative cell death. FASEB J 25:3416–3425
Leitao B, Jones MC, Fusi L, Higham J, Lee Y, Takano M, Goto T, Christian M, Lam EW, Brosens JJ (2010) Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB 24:1541–1551
Acknowledgments
Supported by grant-in-aid from Scientific Research from the Ministry of Education, Science, and Culture, Japan (no. 23592412) and Saitama Medical University Internal Grant 10-08.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kajihara, T., Brosens, J.J. & Ishihara, O. The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med Mol Morphol 46, 61–68 (2013). https://doi.org/10.1007/s00795-013-0018-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-013-0018-z