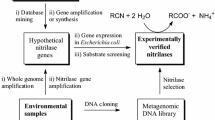
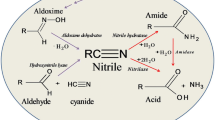
Abstract
Mesophilic nitrile-degrading enzymes are widely dispersed in the Bacteria and lower orders of the eukaryotic kingdom. Two distinct enzyme systems, a nitrilase catalyzing the direct conversion of nitriles to carboxylic acids and separate but cotranscribed nitrile hydratase and amidase activities, are now well known. Nitrile hydratases are metalloenzymes, incorporating FeIII or CoII ions in thiolate ligand networks where they function as Lewis acids. In comparison, nitrilases are thiol-enzymes and the two enzyme groups have little or no apparent sequence or structural homology. The hydratases typically exist as αβ dimers or tetramers in which the α- and β-subunits are similar in size but otherwise unrelated. Nitrilases however, are usually found as homomultimers with as many as 16 subunits. Until recently, the two nitrile-degrading enzyme classes were clearly separated by functional differences, the nitrile hydratases being aliphatic substrate specific and lacking stereoselectivity, whereas the nitrilases are enantioselective and aromatic substrate specific. The recent discovery of novel enzymes in both classes (including thermophilic representatives) has blurred these functional distinctions. Purified mesophilic nitrile-degrading enzymes are typically thermolabile in buffered solution, rarely withstanding exposure to temperatures above 50°C without rapid inactivation. However, operational thermostability is often increased by addition of aliphatic acids or by use of immobilized whole cells. Low molecular stability has frequently been cited as a reason for the limited industrial application of "nitrilases"; such statements notwithstanding, these enzymes have been successfully applied for more than a decade to the kiloton production of acrylamide and more recently to the smaller-scale production of nicotinic acid, R-(−)-mandelic acid and S-(+)-ibuprofen. There is also a rapidly growing catalog of other potentially useful conversions of complex nitriles in which the regioselectivity of the enzyme coupled with the ability to achieve high conversion efficiencies without detriment to other sensitive functionalities is a distinct process advantage.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: January 22, 1998 / Accepted: February 16, 1998
Rights and permissions
About this article
Cite this article
Cowan, D., Cramp, R., Pereira, R. et al. Biochemistry and biotechnology of mesophilic and thermophilic nitrile metabolizing enzymes. Extremophiles 2, 207–216 (1998). https://doi.org/10.1007/s007920050062
Issue Date:
DOI: https://doi.org/10.1007/s007920050062