Abstract
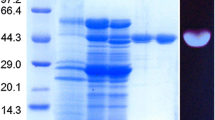
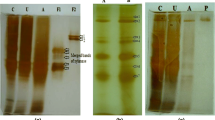
Thermostable alkaline cellulase (endo-1,4-β-glucanase, EC 3.2.1.4) activity was detected in the culture medium of a strictly alkaliphilic strain of Bacillus, designated KSM-S237. This novel enzyme was purified to homogeneity by a two-step column-chromatographic procedure with high yield. The N-terminal amino acid sequence of the purified enzyme was Glu-Gly-Asn-Thr-Arg-Glu-Asp-Asn-Phe-Lys-His-Leu-Leu-Gly-Asn-Asp-Asn-Val-Lys-Arg. The enzyme had a molecular mass of approximately 86 kDa and an isoelectric point of pH 3.8. The enzyme had a pH optimum of 8.6–9.0 and displayed maximum activity at 45°C. The alkaline enzyme was stable up to 50°C and more than 30% of the original activity was detectable after heating at 100°C and at pH 9.0 for 10 min. The enzyme hydrolyzed carboxymethylcellulose, lichenan (β-1,3;1,4-linkage), and p-nitrophenyl derivatives of cellotriose and cellotetraose. Crystalline forms of cellulose (Avicel and filter paper), H3PO4-swollen cellulose, NaOH-swollen cellulose, curdlan (β-1,3-linkage), laminarin (β-1,3;1,6-linkage), and xylan were barely hydrolyzed at all.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: April 28, 1997 / Accepted: May 24, 1997
Rights and permissions
About this article
Cite this article
Hakamada, Y., Koike, K., Yoshimatsu, T. et al. Thermostable alkaline cellulase from an alkaliphilic isolate, Bacillus sp. KSM-S237 . Extremophiles 1, 151–156 (1997). https://doi.org/10.1007/s007920050028
Issue Date:
DOI: https://doi.org/10.1007/s007920050028