Abstract
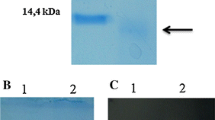
A mangano-superoxide dismutase (EC 1.15.1.1) was purified to homogeneity from a strain of alkaliphilic Bacillus for the first time. The purified protein, with an isoelectric point of pH 4.5, had a molecular mass of approximately 50 kDa and consisted of two identical subunits (25 kDa). The N-terminal amino acid sequence was Ala-Tyr-Lys-Leu-Pro-Glu-Leu-Pro-Tyr-Ala-Ala-Asn-Ala-Leu-Glu-Pro-His-Ile-Asp-Glu-Ala. The optimum pH and temperature for the reaction were 7.5 and 35°C, respectively. The properties of the superoxide dismutase were compared with those of the enzyme from thermophilic Bacillus stearothermophilus.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: September 3, 1996 / Accepted: October 4, 1996
Rights and permissions
About this article
Cite this article
Hakamada, Y., Koike, K., Kobayashi, T. et al. Purification and properties of mangano-superoxide dismutase from a strain of alkaliphilic Bacillus . Extremophiles 1, 74–78 (1997). https://doi.org/10.1007/s007920050017
Issue Date:
DOI: https://doi.org/10.1007/s007920050017