Abstract
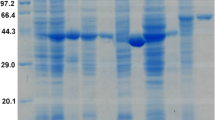
The gene encoding a thermostable iron-containing alcohol dehydrogenase from Thermococcus Strain ES1 (ES1 ADH) was cloned, sequenced and expressed in Escherichia coli. The recombinant and native ES1 ADHs were purified using multistep column chromatography under anaerobic conditions. Both enzymes appeared to be homotetramers with a subunit size of 45 ± 1 kDa as revealed by SDS-PAGE, which was close to the calculated value (44.8 kDa). The recombinant ADH contained 1.0 ± 0.1 g-atom iron per subunit. Both enzymes were sensitive to oxygen with a half-life upon exposure to air of about 4 min. The recombinant enzyme exhibited a specific activity of 105 ± 2 U mg−1, which was very similar to that of the native enzyme (110 ± 3 U mg−1). The optimal pH-values for both enzymes for ethanol oxidation and acetaldehyde reduction were 10.4 and 7.0, respectively. Both enzymes also showed similar temperature-dependent activities, and catalyzed the oxidation of primary alcohols, but there was no activity towards methanol and secondary alcohols. Kinetic parameters of the enzymes showed lower K m-values for acetaldehyde and NADPH and higher K m-values for ethanol and NADP+. It is concluded that the gene encoding ES1 ADH was expressed successfully in E. coli. This is the first report of a fully active recombinant version of an iron-containing ADH from a hyperthermophile.






Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Antoine E, Rolland JL, Raffin JP, Dietrich J (1999) Cloning and over-expression in Escherichia coli of the gene encoding NADPH group III alcohol dehydrogenase from Thermococcus hydrothermalis. Eur J Biochem 264:880–889
Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer EL (2002) The Pfam protein families database. Nucleic Acids Res 30:276–280
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cannio R, Fiorentino G, Carpinelli P, Rossi M, Bartolucci S (1996) Cloning and overexpression in Escherichia coli of the genes encoding NAD-dependent alcohol dehydrogenase from two Sulfolobus species. J Bacteriol 178:301–305
Conway T, Ingram LO (1989) Similarity of Escherichia coli propanediol oxidoreductase (fucO product) and an unusual alcohol dehydrogenase from Zymomonas mobilis and Saccharomyces cerevisiae. J Bacteriol 171:3754–3759
Delano WL (2002) The PyMOL molecular graphics system. Delano Scientific, Palo Alto
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy Server. In: Walker JM (ed) The proteomics protocols handbook. Humana Press, Totowa, pp 571–607
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modelling. Electrophoresis 18:2714–2723
Hirakawa H, Kamiya N, Kawarabayashi Y, Nagamune T (2004) Properties of an alcohol dehydrogenase from the hyperthermophilic archaeon Aeropyrum pernix K1. J Biosci Bioeng 97:202–206
Holland-Staley CA, Lee K, Clark DP, Cunningham PR (2000) Aerobic activity of Escherichia coli alcohol dehydrogenase is determined by a single amino acid. J Bacteriol 182:6049–6054
Hummel W (1999) Large-scale applications of NAD(P)-dependent oxidoreductases: recent developments. Trends Biotechnol 17:487–492
Klages KU, Morgan HW (1994) Characterization of an extremely thermophilic sulphur-metabolizing archaebacterium belonging to the Thermococcales. Arch Microbiol 162:261–266
Kopp J, Schwede T (2004) The SWISS-MODEL repository of annotated three-dimensional protein structure homology models. Nucleic Acids Res 32:D230–D234
Kube J, Brokamp C, Machielsen R, van der Oost J, Märkl H (2006) Influence of temperature on the production of an archaeal thermoactive alcohol dehydrogenase from Pyrococcus furiosus with recombinant Escherichia coli. Extremophiles 10:221–227
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Leary JJ, Brigati DJ, Ward DC (1983) Rapid and sensitive colorimetric method for visualization of biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: bioblots. Proc Natl Acad Sci USA 80:4045–4049
Li D, Stevenson KJ (1997) Purification and sequence analysis of a novel NADP(H)-dependent type III alcohol dehydrogenase from Thermococcus strain AN1. J Bacteriol 179:4433–4437
Lu Z, Cabiscol E, Obradors N, Tamarit J, Ros J, Aguilar J, Lin ECC (1998) Evolution of an Escherichia coli protein with increased resistance to oxidative stress. J Biol Chem 273:8308–8316
Ma K, Adams MWW (1999) An unusual oxygen-sensitive, iron- and zinc-containing alcohol dehydrogenase from the hyperthermophilic archaeon, Pyrococcus furiosus. J Bacteriol 181:1163–1170
Ma K, Adams MWW (2001) Alcohol dehydrogenases from Thermococcus litoralis and Thermococcus strain ES-1. Meth Enzymol 331:195–201
Ma K, Loessner H, Heider J, Johnson MK, Adams MWW (1995) Effects of elemental sulfur on the metabolism of the deep-sea hyperthermophilic archaeon Thermococcus strain ES-1: characterization of a sulfur-regulated, non-heme iron alcohol dehydrogenase. J Bacteriol 177:4748–4756
Ma K, Robb FT, Adams MWW (1994) Purification and characterization of NADP-specific alcohol dehydrogenase and glutamate dehydrogenase from the hyperthermophilic archaeon Thermococcus litoralis. Appl Environ Microbiol 60:562–568
Machielsen R, Uria AR, Kengen SWM, van der Oost J (2006) Production and characterization of a thermostable alcohol dehydrogenase that belongs to the aldo-keto reductase superfamily. Appl Environ Microbiol 72:233–238
Montella C, Bellsolell L, Pérez-Luque R, Badía J, Baldoma L, Coll M, Aguilar J (2005) Crystal structure of an iron-dependent group III dehydrogenase that interconverts l-lactaldehyde and l-1,2-propanediol in Escherichia coli. J Bacteriol 187:4957–4966
Neale AD, Scopes RK, Kelly JM, Wettenhall RE (1986) The two alcohol dehydrogenases of Zymomonas mobilis. Purification by differential dye ligand chromatography, molecular characterisation and physiological roles. Eur J Biochem 154:119–124
Neuner A, Jannasch HW, Belkin S, Stetter KO (1989) Thermococcus litoralis sp. nov.: a new species of extremely thermophilic marine archaebacteria. Arch Microbiol 153:205–207
Peitsch MC (1995) Protein modeling by E-mail. Bio Technol 13:658–660
Pledger RJ, Baross J (1989) Characterization of an extremely thermophilic archaebacterium isolated from a black smoker polychaete (Paralvinella, sp.) at the Juan de Fuca Ridge. Syst Appl Microbiol 12:249–256
Reid MF, Fewson CA (1994) Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol 20:13–56
Ronimus RS, Reysenbach AL, Musgrave DR, Morgan HW (1997) The phylogenetic position of the Thermococcus isolate AN1 based on 16S rRNA gene sequence analysis: a proposal that AN1 represents a new species, Thermococcus zilligii sp. nov. Arch Microbiol 168:245–248
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schwarzenbacher R, von Delft F, Canaves JM, Brinen LS, Dai X, Deacon AM, Elsliger MA, Eshaghi S, Floyd R, Godzik A, Grittini C, Grzechnik SK, Guba C, Jaroszewski L, Karlak C, Klock HE, Koesema E, Kovarik JE, Kreusch A, Kuhn P, Lesley SA, McMullan D, McPhillips TM, Miller MA, Miller MD, Morse A, Moy K, Ouyang J, Page R, Robb A, Rodrigues K, Selby TL, Spraggon G, Stevens RC, van den Bedem H, Velasquez J, Vincent J, Wang X, West B, Wolf G, Hodgson KO, Wooley J, Wilson IA (2004) Crystal structure of an iron-containing 1,3-propanediol dehydrogenase (TM0920) from Thermotoga maritima at 1.3 Å resolution. Proteins 54:174–177
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Stetter KO (1988) Hyperthermophiles–physiology and enzymes. J Chem Technol Biotechnol 42:315–317
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Unden G, Becker S, Bongaerts J, Schirawski J, Six S (1994) Oxygen regulated gene expression in facultatively anaerobic bacteria. Antonie Leeuwenhoek 66:3–23
van der Oost J, Voorhorst WGB, Kengen SWM, Geerling ACM, Wittenhorst V, Gueguen Y, de Vos WM (2001) Genetic and biochemical characterization of a short-chain alcohol dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur J Biochem 268:3062–3068
Walter KA, Bennett GN, Papoutsakis ET (1992) Molecular characterization of two Clostridium acetobutylicum ATCC 824 butanol dehydrogenase isozyme genes. J Bacteriol 174:7149–7158
Ying X (2007) Characterization of iron- and zinc-containing alcohol dehydrogenases from anaerobic hyperthermophiles. PhD Thesis, University of Waterloo, Waterloo, Canada. http://hdl.handle.net/10012/3446
Ying X, Wang Y, Badiei HR, Karanassios V, Ma K (2007) Purification and characterization of an iron-containing alcohol dehydrogenase in extremely thermophilic bacterium Thermotoga hypogea. Arch Microbiol 187:499–510
Zheng Y, Cao Y, Fang B (2004) Cloning and sequence analysis of the dhaT gene of the 1,3-propanediol regulon from Klebsiella pneumoniae. Biotechnol Lett 26:251–255
Acknowledgments
This work was supported by research grants from Natural Sciences and Engineering Research Council (Canada), Ontario Ministry of Agriculture and Food—Rural Affairs and Canada Foundation for Innovation to KM, and by a grant (BES-061723) from the US National Science Foundation to MA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Santos.
Rights and permissions
About this article
Cite this article
Ying, X., Grunden, A.M., Nie, L. et al. Molecular characterization of the recombinant iron-containing alcohol dehydrogenase from the hyperthermophilic Archaeon, Thermococcus strain ES1. Extremophiles 13, 299–311 (2009). https://doi.org/10.1007/s00792-008-0217-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-008-0217-z