Abstract
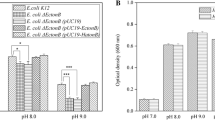
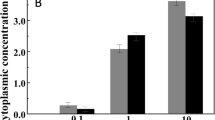
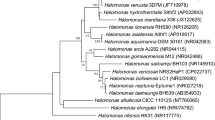
Halobacterium species balance high external osmolality by the accumulation of almost equimolar amounts of KCl. Thus, steady K+ supply is a vital prerequisite for life of these extreme halophiles. So far, K+ is reported to enter the halobacterial cell only passively by use of potential-driven uniporters. However, the genome of both the extreme halophilic archaeon Halobacterium sp. NRC-1 and H. salinarum R1 comprises one single gene cluster containing the genes kdpFABC coding for homologs of the bacterial ATP-driven K+ uptake system KdpFABC, together with an additional ORF so far annotated as cat3 in Halobacterium sp. NRC-1 and as UspA protein in H. salinarum R1 (the ORF is only referred to as cat3 in the following). Deletion of the kdpFABCcat3 genes led to a reduced ability to grow under limiting K+ concentrations, whereas real-time RT-PCR measurements revealed cat3-dependent high expression rates of the Kdp system in case of external K+ depletion. Synthesis of the KdpFABC complex enables H. salinarum R1 to grow under extreme potassium-limiting conditions of >20 μM K+. These results provide the first experimental evidence of an ATP-driven K+ uptake system in Halobacterium. Moreover, H. salinarum R1 was shown to further adapt to K+ limitation by a significant decrease of the intracellular K+ level, which suggests a rather complex mechanism of K+ homeostasis, in which the adaptation of cellular K+ concentrations and the concomitant transcriptional regulation of genes coding for a high-affinity ATP-driven K+ uptake system ensure the essential potassium supply under limiting conditions.








Similar content being viewed by others
Abbreviations
- BRE:
-
Transcription factor B responsive element
- FEP:
-
Flame emission photometry
- INR:
-
Transcription initiation region
- OD:
-
Optical density
- ORF:
-
Open reading frame
- RT:
-
Reverse transcriptase
- TFB:
-
Transcription factor B
- Usp:
-
Universal stress protein
- ΔψK+ :
-
Potassium potential
- Δψm :
-
Membrane potential
References
Aravind L, Anantharaman V, Koonin EV (2002) Monophyly of class I aminoacyl tRNA synthetase, USPA, ETFP, photolyase, and PP-ATPase nucleotide-binding domains: implications for protein evolution in the RNA. Proteins 48:1–14
Bakker EP (1993) Low-affinity K+ uptake systems. In: Bakker EP (ed) Alkali cation transport systems in prokaryotes. CRC Press Inc, Boca Raton, pp 253–276
Baliga NS, DasSarma S (1999) Saturation mutagenesis of the TATA box and upstream activator sequence in the haloarchaeal bop gene promoter. J Bacteriol 181:2513–2518
Baliga NS, Kennedy SP, Ng WV, Hood L, DasSarma S (2001) Genomic and genetic dissection of an archaeal regulon. Proc Natl Acad Sci USA 98:2521–2525
Bramkamp M, Altendorf K, Greie J-C (2007) Common patterns and unique features of P-type ATPases: a comparative view on the KdpFABC complex from Escherichia coli. Mol Membr Biol 24:375–386
Brenneis M, Hering O, Lange C, Soppa J (2007) Experimental characterization of Cis-acting elements important for translation and transcription in halophilic archaea. PLoS Genet 3:e229
Cline SW, Lam WL, Charlebois RL, Schalkwyk LC, Doolittle WF (1989) Transformation methods for halophilic archaebacteria. Can J Microbiol 35:148–152
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D et al (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544
Elcock AH, McCammon JA (1998) Electrostatic contributions to the stability of halophilic proteins. J Mol Biol 24:731–748
Greie J-C, Altendorf K (2007) The K+-translocating KdpFABC complex from Escherichia coli: a P-type ATPase with unique features. J Bioenerg Biomembr 39:397–402
Gropp F, Gropp R, Betlach MC (1995) Effects of upstream deletions on light- and oxygen-regulated bacterio-opsin gene expression in Halobacterium halobium. Mol Microbiol 16:357–364
Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J Bacteriol 177:4121–4130
Heermann R, Altendorf K, Jung K (2000) The hydrophilic N-terminal domain complements the membrane-anchored C-terminal domain of the sensor kinase KdpD of Escherichia coli. J Biol Chem 275:17080–17085
Jung K, Altendorf K (2002) Towards an understanding of the molecular mechanisms of stimulus perception and signal transduction by the KdpD/KdpE system of Escherichia coli. J Mol Microbiol Biotechnol 4:223–228
Kauri T, Wallace R, Kushner DJ (1990) Nutrition of the halophilic archaebacterium, Haloferax volcanii. Syst Appl Microbiol 13:14–18
Kennedy SP, Ng WV, Salzberg SL, Hood L, DasSarma S (2001) Understanding the adaptation of Halobacterium species NRC-1 to its extreme environment through computational analysis of its genome sequence. Genome Res 11:1641–1650
Klenk HP, Clayton RA, Tomb J-F, White O, Nelson KE, Ketchum KA et al (1997) The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature 390:364–370
Koch MK, Oesterhelt D (2005) MpcT is the transducer for membrane potential changes in Halobacterium salinarum. Mol Microbiol 55:1681–1694
Kokoeva MV, Storch KF, Klein C, Oesterhelt D (2002) A novel mode of sensory transduction in archaea: binding protein-mediated chemotaxis towards osmoprotectants and amino acids. EMBO J 15:2312–2322
Krebs MP, Mollaaghababa R, Khorana HG (1993) Gene replacement in Halobacterium halobium and expression of bacteriorhodopsin mutants. Proc Natl Acad Sci USA 90:1987–1991
Lanyi JK (1978) Light energy conversion in Halobacterium halobium. Microbiol Rev 42:682–706
Lanyi JK, Helgerson SL, Silverman MP (1979) Relationship between proton motive force and potassium ion transport in Halobacterium halobium envelope vesicles. Arch Biochem Biophys 193:329–339
Madern D, Ebel C, Zaccai G (2000) Halophilic adaptation of enzymes. Extremophiles 4:91–98
Meury J, Kohiyama M (1989) ATP is required for K+ active transport in the archaebacterium Haloferax volcanii. Arch Microbiol 151:530–536
Michel H, Oesterhelt D (1980) Electrochemical proton gradient across the cell membrane of Halobacterium halobium: effect of N,N′-dicyclohexylcarbodiimide, relation to intracellular adenosine triphosphate, adenosine diphosphate, and phosphate concentration, and influence of the potassium gradient. Biochemistry 19:4607–4614
Nakamura T, Yamamuro N, Stumpe S, Bakker EP (1998) Cloning of the trkAH gene cluster and characterization of the Trk K+-uptake system of Vibrio alginolyticus. Microbiology 144:2281–2289
Ng WV, Kennedy SP, Mahairas GG, Berquist B, Pan M, Shukla HD et al (2000) Genome sequence of Halobacterium species NRC-1. Proc Natl Acad Sci USA 97:12176–12181
Oren A (1999) Bioenergetic aspects of halophilism. Microbiol Mol Biol Rev 63:334–348
Palmer JR, Daniels CJ (1995) In vivo definition of an archaeal promoter. J Bacteriol 177:1844–1849
Palmgren MG, Axelsen KB (1998) Evolution of P-type ATPases. Biochim Biophys Acta 1365:37–45
Pfeiffer F, Schuster SC, Broicher A, Falb M, Palm P, Rodewald K, Ruepp A et al (2008) Evolution in the laboratory: the genome of Halobacterium salinarum strain R1 compared to that of strain NRC-1. Genomics 91:335–346
Qureshi SA, Jackson SP (1998) Sequence-specific DNA binding by the S. shibatae TFIIB homolog, TFB, and its effect on promoter strength. Mol Cell 1:389–400
Roberts MF (2005) Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Systems 1:5
Sambrook J, Fritsch EF, Maniatis T (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Siebers A, Altendorf K (1993) K+-translocating Kdp-ATPases and other bacterial P-type ATPases. In: Bakker EP (ed) Alkali cation transport systems in prokaryotes. CRC Press Inc, Boca Raton, pp 225–252
Simon P (2003) Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 22:1439–1440
Soppa J (1999) Transcription initiation in Archaea: facts, factors and future aspects. Mol Microbiol 31:1295–1305
Stoeckenius W, Kunau WH (1968) Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J Cell Biol 38:337–357
Stumpe S, Schlösser A, Schleyer M, Bakker EP (1996) K+ circulation across prokaryotic cell membrane: K+ uptake systems. In: Konings WN, Kaback HR, Lolkema JS (eds) Handbook of biological physics, vol 2. Elsevier, Amsterdam, pp 473–499
Wagner G, Hartmann R, Oesterhelt D (1978) Potassium uniport and ATP synthesis in Halobacterium halobium. Eur J Biochem 89:169–179
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 87:4576–4579
Acknowledgments
The authors kindly acknowledge Karlheinz Altendorf as well as the Arbeitsgruppe Mikrobiologie (Universität Osnabrück) for generous scientific and financial support. Ursula Krehe is acknowledged for excellent technical assistance. Dieter Oesterhelt and Jörg Tittor are kindly acknowledged for valuable scientific discussion as well as for the kind donation of plasmid pMKK100. H. S. was supported by the Boehringer Ingelheim Fonds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Robb.
Rights and permissions
About this article
Cite this article
Strahl, H., Greie, JC. The extremely halophilic archaeon Halobacterium salinarum R1 responds to potassium limitation by expression of the K+-transporting KdpFABC P-type ATPase and by a decrease in intracellular K+ . Extremophiles 12, 741–752 (2008). https://doi.org/10.1007/s00792-008-0177-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-008-0177-3