Abstract
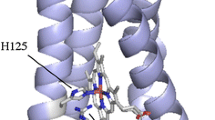
Cys-59 and Cys-62, forming a disulfide bond in the four-residue loop of Shewanella violacea cytochrome c 5 (SV cytc 5), contribute to protein stability but not to redox function. These Cys residues were substituted with Ala in SV cytc 5, and the structural and functional properties of the resulting C59A/C62A variant were determined and compared with those of the wild-type. The variant had similar features to those of the wild-type in absorption, circular dichroic, and paramagnetic 1H NMR spectra. In addition, the redox potentials of the wild-type and variant were essentially the same, indicating that removal of the disulfide bond from SV cytc 5 does not affect the redox function generated in the vicinity of heme. However, calorimetric analysis of the wild-type and variant showed that the mutations caused a drastic decrease in the protein stability through enthalpy, but not entropy. Four residues are encompassed by the SV cytc 5 disulfide bond, which is the shortest one that has been proved to affect protein stability. The protein stability of SV cytc 5 can be controlled without changing the redox function, providing a new strategy for regulating the stability and function of cytochrome c.






Similar content being viewed by others
Abbreviations
- SV cytc 5 :
-
Shewanella violacea mono-heme cytochrome c5
- CD:
-
Circular dichroism
- CV:
-
Cyclic voltammetry
- DSC:
-
Differential scanning calorimetry
- PCR:
-
Polymerase chain reaction
- SDS:
-
Sodium dodecyl sulfate
References
Akiyama Y, Kamitani S, Kusukawa N, Ito K (1992) In vitro catalysis of oxidative folding of disulfide-bonded proteins by the Escherichia coli dsbA (ppfA) gene product. J Biol Chem 267:22440–22445
Ambler RP (1991) Sequence variability in bacterial cytochromes c. Biochim Biophys Acta 1058:42–47
Arslan E, Schulz H, Zufferey R, Künzler P, Thöny-Meyer L (1998) Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb 3 oxidase in Escherichia coli. Biochem Biophys Res Commun 251:744–747
Bartalesi I, Bertini I, Hajieva P, Rosato A, Vasos PR (2002) Solution structure of a monoheme ferrocytochrome c from Shewanella putrefaciens and structural analysis of sequence-similar proteins: functional implications. Biochemistry 41:5112–5119
Berry EA, Huang LS, Saechao LK, Pon NP, Valkova-Valchanova M, Daldal F (2004) X-ray structure of Rhodobacter capsulatus cytochrome bc 1: comparison with its mitochondrial and chloroplast counterparts. Photosynth Res 81:251–275
Bertini I, Cavallaro G, Rosato A (2006) Cytochrome c: occurrence and functions. Chem Rev 106:90–115
Betz SF (1993) Disulfide bonds and the stability of globular proteins. Protein Sci 2:1551–1558
Bonander N, Leckner J, Guo H, Karlsson BG, Sjölin L (2000) Crystal structure of the disulfide bond-deficient azurin mutant C3A/C26A: how important is the S–S bond for folding and stability? Eur J Biochem 267:4511–4519
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brems DN, Cass R, Stellwagen E (1982) Conformational transitions of frog heart ferricytochrome c. Biochemistry 21:1488–1493
Carter DC, Melis KA, O’Donnell SE, Burgess BK, Furey WR Jr, Wang BC, Stout CD (1985) Crystal structure of Azotobacter cytochrome c 5 at 2.5 Å resolution. J Mol Biol 184:279–295
Chida H, Yokoyama T, Kawai F, Nakazawa A, Akazaki H, Takayama Y, Hirano T, Suruga K, Satoh T, Yamada S, Kawachi R, Unzai S, Nishio T, Park SY, Oku T (2006) Crystal structure of oxidized cytochrome c 6A from Arabidopsis thaliana. FEBS Lett 580:3763–3768
Doig AJ, Williams DH (1991) Is the hydrophobic effect stabilizing or destabilizing in proteins? The contribution of disulphide bonds to protein stability. J Mol Biol 217:389–398
Elberry M, Yu L, Yu CA (2006) The disulfide bridge in the head domain of Rhodobacter sphaeroides cytochrome c 1 is needed to maintain its structural integrity. Biochemistry 45:4991–4997
Esser L, Gong X, Yang S, Yu L, Yu CA, Xia D (2006) Surface-modulated motion switch: capture and release of iron-sulfur protein in the cytochrome bc 1 complex. Proc Natl Acad Sci USA 103:13045–13050
Feige MJ, Hagn F, Esser J, Kessler H, Buchner J (2007) Influence of the internal disulfide bridge on the folding pathway of the CL antibody domain. J Mol Biol 365:1232–1244
Goodhew CF, Brown KR, Pettigrew GW (1986) Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes. Biochim Biophys Acta 852:288–294
Goto Y, Hamaguchi K (1982) Unfolding and refolding of the reduced constant fragment of the immunoglobulin light chain. Kinetic role of the intrachain disulfide bond. J Mol Biol 156:911–926
Guzzi R, Sportelli L, La Rosa C, Milardi D, Grasso D, Verbeet MP, Canters GW (1999) A spectroscopic and calorimetric investigation on the thermal stability of the Cys3Ala/Cys26Ala azurin mutant. Biophys J 77:1052–1063
Hasegawa J, Shimahara H, Mizutani M, Uchiyama S, Arai H, Ishii M, Kobayashi Y, Ferguson SJ, Sambongi Y, Igarashi Y (1999) Stabilization of Pseudomonas aeruginosa cytochrome c 551 by systematic amino acid substitutions based on the structure of thermophilic Hydrogenobacter thermophilus cytochrome c 552. J Biol Chem 274:37533–37537
Kidera A, Inaka K, Matsushima M, Go N (1994) Response of dynamic structure to removal of a disulfide bond: normal mode refinement of C77A/C95A mutant of human lysozyme. Protein Sci 3:92–102
Kuroki R, Inaka K, Taniyama Y, Kidokoro S, Matsushima M, Kikuchi M, Yutani K (1992) Enthalpic destabilization of a mutant human lysozyme lacking a disulfide bridge between cysteine-77 and cysteine-95. Biochemistry 31:8323–8328
Marcaida MJ, Schlarb-Ridley BG, Worrall JAR, Wastl J, Evans TJ, Bendall DS, Luisi BF, Howe CJ (2006) Structure of cytochrome c 6A, a novel dithio-cytochrome of Arabidopsis thaliana, and its reactivity with plastocyanin: implications for function. J Mol Biol 360:968–977
Moore GR, Pettigrew GW (1990) Cytochromes c—evolutionary, structural and physicochemical aspects. Springer, Heidelberg
Nogi Y, Kato C, Horikoshi K (1998) Taxonomic studies of deep-sea barophilic Shewanella strains and description of Shewanella violacea sp. nov. Arch Microbiol 170:331–338
Oikawa K, Nakamura S, Sonoyama T, Ohshima A, Kobayashi Y, Takayama SJ, Yamamoto Y, Uchiyama S, Hasegawa J, Sambongi Y (2005) Five amino acid residues responsible for the high stability of Hydrogenobacter thermophilus cytochrome c 552: reciprocal mutation analysis. J Biol Chem 280:5527–5532
Osyczka A, Dutton PL, Moser CC, Darrouzet E, Daldal F (2001) Controlling the functionality of cytochrome c 1 redox potentials in the Rhodobacter capsulatus bc 1 complex through disulfide anchoring of a loop and a beta-branched amino acid near the heme-ligating methionine. Biochemistry 40:14547–14556
Pace CN (1990) Measuring and increasing protein stability. Trends Biotechnol 8:93–98
Pace CN, Grimsley GR, Thomson JA, Barnett BJ (1988) Conformational stability and activity of ribonuclease T1 with zero, one, and two intact disulfide bonds. J Biol Chem 263:11820–11825
Reid KSC, Lindley PF, Thornton JM (1985) Sulfur–aromatic interactions in proteins. FEBS Lett 190:209–213
Sambongi Y, Stoll R, Ferguson SJ (1996) Alteration of haem-attachment and signal-cleavage sites for Paracoccus denitrificans cytochrome c 550 probes pathway of c-type cytochrome biogenesis in Escherichia coli. Mol Microbiol 19:1193–1204
Sambongi Y, Uchiyama S, Kobayashi Y, Igarashi Y, Hasegawa J (2002) Cytochrome c from a thermophilic bacterium has provided insights into the mechanisms of protein maturation, folding, and stability. Eur J Biochem 269:3355–3361
Swift TJ (1973) Principles and applications, In: La Mar GN, Horrocks W, DeW Jr, Holm RH (eds) NMR of paramagnetic molecules. Academic, New York, pp 53–83
Terui N, Tachiiri N, Matsuo H, Hasegawa J, Uchiyama S, Kobayashi Y, Igarashi Y, Sambongi Y, Yamamoto Y (2003) Relationship between redox function and protein stability of cytochromes c. J Am Chem Soc 125:13650–13651
Uchiyama S, Hasegawa J, Tanimoto Y, Moriguchi H, Mizutani M, Igarashi Y, Sambongi Y, Kobayashi Y (2002) Thermodynamic characterization of variants of mesophilic cytochrome c and its thermophilic counterpart. Protein Eng 15:455–461
Uchiyama S, Ohshima A, Nakamura S, Hasegawa J, Terui N, Takayama SJ, Yamamoto Y, Sambongi Y, Kobayashi Y (2004) Complete thermal-unfolding profiles of oxidized and reduced cytochromes c. J Am Chem Soc 126:14684–14685
Vogl T, Brengelmann R, Hinz HJ, Scharf M, Lötzbeyer M, Engels JW (1995) Mechanism of protein stabilization by disulfide bridges: calorimetric unfolding studies on disulfide-deficient mutants of the α-amylase inhibitor tendamistat. J Mol Biol 254:481–496
Wastl J, Molina-Heredia FP, Hervás M, Navarro JA, De la Rosa MA, Bendall DS, Howe CJ (2004) Redox properties of Arabidopsis cytochrome c 6 are independent of the loop extension specific to higher plants. Biochim Biophys Acta 1657:115–120
Williams P, Coates L, Mohammed F, Gill R, Erskine P, Bourgeois D, Wood SP, Anthony C, Cooper JB (2006) The 1.6 Å X-ray structure of the unusual c-type cytochrome, cytochrome c L, from the methylotrophic bacterium Methylobacterium extorquens. J Mol Biol 357:151–162
Yamada M, Nakasone K, Tamegai H, Kato C, Usami R, Horikoshi K (2000) Pressure regulation of soluble cytochromes c in a deep-sea piezophilic bacterium, Shewanella violacea. J Bacteriol 182:2945–2952
Zhang Y, Arai H, Sambongi Y, Igarashi Y, Kodama T (1998) Heterologous expression of Hydrogenobacter thermophilus cytochrome c-552 in the periplasm of Pseudomonas aeruginosa. J Ferment Bioeng 85:346–349
Acknowledgments
This work was financially supported in part by grants from the Japanese Ministry of Education, Science and Culture (Grants-in-Aid for Scientific Research on Priority Areas) to Y. S. and Y.Y. (17350081), the Yazaki Memorial Foundation for Science and Technology, and the NOVARTIS Foundation (Japan) for the Promotion of Science to Y.Y.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi.
Rights and permissions
About this article
Cite this article
Ogawa, K., Sonoyama, T., Takeda, T. et al. Roles of a short connecting disulfide bond in the stability and function of psychrophilic Shewanella violacea cytochrome c 5*. Extremophiles 11, 797–807 (2007). https://doi.org/10.1007/s00792-007-0099-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-007-0099-5