Abstract
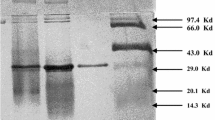
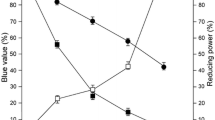
A gene that encodes the enzyme Pyrococcus furiosus cyclodextrin glucanotransferase (PFCGT) was cloned in Escherichia coli. PFCGT was highly expressed in recombinant E. coli after compensation for codon usage bias using the pRARE plasmid. Purified PFCGT was extremely thermostable with an optimal temperature and pH of 95°C and 5.0, respectively, retaining 97% of its activity at 100°C. Incubation at 60°C for 20 min during the purification process led to a 1.5-fold increase in enzymatic activity. A time course assay of the PFCGT reaction with starch indicated that cyclic α-1,4-glucans with DPs greater than 20 were produced at the beginning of the incubation followed by an increase in β-CD. The major final product of PFCGT cyclization was β-CD, and thus the enzyme is a β-CGTase.



Similar content being viewed by others
References
Bibel M, Brettl C, Gosslar U, Kriegshäuser G, Liebl W (1998) Isolation and analysis of genes for amylolytic enzymes of the hyperthermophilic bacterium Thermotoga maritima. FEMS Microbiol Lett 158:9–15
Dong G, Vieille C, Savchenko A, Zeikus JG (1997a) Cloning, sequencing, and expression of the gene encoding extracellular α-amylase from Pyrococcus furiosus and biochemical characterization of the recombinant enzyme. Appl Environ Microbiol 63:3569–3576
Dong G, Vieille C, Zeikus JG (1997b) Cloning, sequencing, and expression of the gene encoding amylopullulanase from Pyrococcus furiosus and biochemical characterization of the recombinant enzyme. Appl Environ Microbiol 63:3577–3584
Kaneko T, Kato T, Nakamura N, Horikoshi K (1987) Spectrophotometric determination of cyclization activity of β-cyclodextrin-forming cyclomaltodextrin glucanotransferase. J Jpn Soc Starch Sci 34:45–48
Kim YW, Lee SS, Warren RAJ, Withers SG (2004) Directed evolution of a glycosynthase from Agrobacterium sp. increases its catalytic activity dramatically and expands its substrate repertoire. J Biol Chem 279:42787–42793
Nelson KE, Clayton RA, Gill SR, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Nelson WC, Ketchum KA, McDonald L, Utterback TR, Malek JA, Linher KD, Garrett MM, Stewart AM, Cotton MD, Pratt MS, Phillips CA, Richardson D, Heidelberg J, Sutton GG, Fleischmann RD, Eisen JA, White O, Salzberg SL, Smith HO, Venter JC, Fraser CM (1999) Evidence for lateral gene transfer between archaea and bacteria from genome sequence of Thermotoga maritima. Nature 399:323–329
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Rahman RNZA, Fujiwara S, Takagi M, Kanaya S, Imanaka T (1997) Effect of heat treatment on proper oligomeric structure formation of thermostable glutamate dehydrogenase from a hyperthermophilic archaeon. Biochem Biophys Res Commun 241:646–652
Rahman RNZA, Fujiwara S, Nakamura H, Takagi M, Imanaka T (1998) Ion pairs involved for maintaining a thermostable structure of glutamate dehydrogenase (GDH) from a hyperthermophilic archaeon. Biochem Biophys Res Commun 248:920–926
Rashid N, Cornista J, Ezaki S, Fukui T, Atomi H, Imanaka T (2002) Characterization of an archaeal cyclodextrin glucanotransferase with a novel C-terminal domain. J Bacteriol 184:777–784
Rice DW, Yip KSP, Stillman TJ, Britton KL, Fuentes A, Connerton I, Pasquo A, Acandurra R, Engel PC (1996) Insights into the molecular basis of thermal stability from the structure determination of Pyrococcus furiosus glutamate dehydrogenase. FEMS Microbiol Rev 18:105–117
Rose NN, Li D, Park JT, Cha HJ, Kim MS, Kim JW, Park KH (2005) Characterization and application of a novel thermostable glucoamylase cloned from a hyperthermophilic archaeon Sulfolobus tokodaii. Food Sci Biotechnol 14:860–865
Saenger W (1980) Cyclodextrin inclusion compounds in research and industry. Angew Chem Int Ed 19:344–362
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schiraldi C, Rosa MD (2002) The production of biocatalysts and biomolecules from extremophiles. Trends Biotechnol 20:515–521
Schmid G (1989) Cyclodextrin glycosyltransferase production: yield enhancement by overexpression of cloned genes. Tibtech 7:244–248
Schultes V, Jaenicke R (1991) Folding intermediates of hyperthermophilic D-glyceraldehyde-3-phosphate dehydrogenase from Thermotoga maritima are trapped at low temperature. FEBS Lett 290:235–238
Siddiqui MA, Fujiwara S, Takagi M, Imanaka T (1998) In vitro heat effect on heterooligomeric subunit assembly of thermostable indolepyruvate ferredoxin oxidoreductase. FEBS Lett 434:372–376
Tachibana Y, Leclere MM, Fujiwara S, Takagi M, Imanaka T (1996) Cloning and expression of the α-amylase gene from the hyperthermophilic archaeon Pyrococcus sp. KOD1, and characterization of the enzyme. J Ferment Bioeng 82:224–232
Tachibana Y, Kuramura A, Shirasaka N, Suzuki Y, Yamamoto T, Fujiwara S, Takagi M, Imanaka T (1999) Purification and characterization of an extremely thermostable cyclomaltodextrin glucanotransferase from a newly isolated hyperthermophilic archaeon, a Thermococcus sp. Appl Environ Microbiol 65:1991–1997
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Yang SJ, Lee HS, Park CS, Kim YR, Moon TW, Park KH (2004) Enzymatic analysis of an amylolytic enzyme from the hyperthermophilic archaeon Pyrococcus furiosus reveals its novel catalytic properties as both an α-amylase and a cyclodextrin-hydrolyzing enzyme. Appl Environ Microbiol 70:5988–5995
Acknowledgments
We thank A. M. Grunden of North Carolina State University, USA, for donating the P. furiosus DSM3638 genomic DNA used in the study. This study was supported by the Marine and Extreme Genome Research Center Program, Ministry of Marine Affairs and Fisheries, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi.
Rights and permissions
About this article
Cite this article
Lee, MH., Yang, SJ., Kim, JW. et al. Characterization of a thermostable cyclodextrin glucanotransferase from Pyrococcus furiosus DSM3638. Extremophiles 11, 537–541 (2007). https://doi.org/10.1007/s00792-007-0061-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-007-0061-6