Abstract
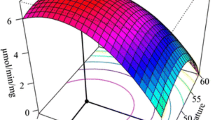
We examined variants of an especially cold-active β-galactosidase (BgaS) to better understand features affecting enzyme activity at temperature extremes. We targeted locations corresponding to a region in the LacZ enzyme previously shown to increase activity and decrease thermostability. Changes in this region of BgaS consistently caused the elimination or reduction of activity. A gene (bgaS3) encoding a loss of function variant was subjected to random mutagenesis to restore activity and discover potential interactions important in cold activity. Gene sequences from the resulting library indicated that only two amino acid alterations, E229D and V405A, were required to restore activity. Genes with combinations of these mutations were constructed and their enzymes purified. Enzymes with the E229D/V405A/G803D alterations (BgaS6), or E229D/V405A (BgaS7) had similar thermal optima and thermostabilities as BgaS. BgaS7, however, showed a 2.5-fold increase in catalytic activity at 15°C and hydrolyzed 80% of lactose in skim milk in less than half the time of BgaS at 2.5°C. Computer-generated models predicted that the substitutions at positions 229 and 405 yielded fewer contacts at the enzyme’s activating interface. Results from regional saturation mutagenesis supported this hypothesis and suggested that not easily predicted, subtle, cooperative intramolecular interactions contributed to thermal adaptation.


Similar content being viewed by others
References
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TH, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242
Coker JA, Sheridan PP, Loveland-Curtze J, Gutshall KR, Auman AJ, Brenchley JE (2003) Biochemical characterization of a β-galactosidase with a low temperature optimum obtained from an Antarctic Arthrobacter isolate. J Bacteriol 185:5473–5482
Dahlquist A (1983) Digestion of lactose. In: Delmont J (eds) Milk intolerance and rejection. Karger, Basel, New York City pp 11–16
D’Amico S, Claverie P, Collins T, Georlette D, Gratia E, Hoyoux A, Meuwis M-A, Feller G, Gerday C (2002) Molecular basis of cold adaptation. Phil Trans R Soc London 357:917–925
Fernandes S, Geueke B, Delgado J, Coleman J, Hatti-Kaul R (2002) β-galactosidase from a cold-adapted bacterium: purification, characterization and application for lactose hydrolysis. Appl Microbiol Biotechnol 58:313–321
Fowler A, Zabin I (1978) Amino acid sequence of beta-galactosidase. J Biol Chem 253:5521–5525
Georlette D, Blaise V, Collins T, D’Amico S, Gratia E, Hoyoux A, Marx J-C, Sonan G, Feller G, Gerday C (2004) Some like it cold: biocatalysis at low temperatures. FEMS Microbiol Rev 28:25–42
Gutshall KR, Trimbur DE, Kasmir JJ, Brenchley JE (1995) Analysis of a novel gene and β-galactosidase isozyme from a psychrotrophic Arthrobacter isolate. J Bacteriol 177:1981–1988
Hennrissat B, Davies G (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637–644
Hoyoux A, Jennes AI, Dubois P, Genicot S, Dubail F, Francois JM, Baise E, Feller G, Gerday C (2001) Cold-adapted β-galactosidase from the Antarctic psychrophile Pseudoalteromonas haloplanktis. Appl Environ Microbiol 67:
Huber RE, Hakda S, Cheng C, Cupples CG, Edwards RA (2003) Trp-999 of β-galactosidase (Escherichia coli) is a key residue for binding, catalysis, and synthesis of allolactose, the natural lac operon inducer. Biochemistry 42:1796–1803
Ishmael FT, Alley SC, Benkovic SJ (2001) Identification and mapping of protein-protein interactions between gp32 and gp59 by cross-linking. J Biol Chem 276:25236–25242
Juers DH, Hakda S, Matthews BW, Huber RE (2003) Structural basis for the altered activity of Gly-794 variants of Escherichia coli β-galactosidase. Biochemistry 42:13505–13511
Juers DH, Heightman TD, Vasella A, McCarter JD, Mackenzie L, Withers SG, Matthews BW (2001) A structural view of the action of Escherichia coli (LacZ) β-galactosidase. Biochemistry 40:14781–14794
Kano H, Taguchi S, Momose H (1997) Cold adaptation of a mesophilic serine protease, subtilisin, by in vitro random mutagenesis. Appl Microbiol Biotechnol 47:46–51
Karasova-Lipovova P, Strnad H, Spiwok V, Mala S, Kralova B, Russell NJ (2003) The cloning, purification and characterisation of a cold-active β-galactosidase from the psychrotolerant Antarctic bacterium Arthrobacter sp. C2-2. Enzyme Microb Technol 33:836–844
Martinez-Bilbao M, Holdsworth RE, Edwards RA, Huber RE (1991) A highly reactive β-galactosidase (Escherichia coli) resulting from a substitution of an aspartic acid for Gly-794. J Biol Chem 266:4979–4986
Martinez-Bilbao M, Huber RE (1994) Substitutions for Gly-794 show that binding interactions are important determinants of the catalytic action of beta-galactosidase (Escherichia coli). Biochem Cell Biol 72:313–319
Merz A, Yee MC, Szadkowski H, Pappenberger G, Crameri A, Stemmer WP (2000) Improving the catalytic activity of a thermophilic enzyme at low temperatures. Biochemistry 39:880–889
Miller J (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor
Nichtl A, Buchner J, Jaenicke R, Rudolph R, Scheibel T (1998) Folding and association of β-galactosidase. J Mol Biol 282:1083–1091
Notredame C, Higgins D, Heringa J (2000) T-Coffee: a novel method for multiple sequence alignments. J Mol Biol 302:205–217
Oikawa T, Yamanaka K, Kazuoka T, Kazuika N, Soda K (2001) Psychrophilic valine dehydrogenase of the Antarctic psychrophile, Cytophaga sp. KUC-1––purification, molecular characterization and expression. Eur J Biochem 268:4375–4383
Panasik Jr. N, Brenchley JE, Farber GK (2000) Distribution of the structural features contributing to thermostability in mesophilic (α/β)8 barrel glycosyl hydrolases. Biochim Biophys Acta 1543:189–201
Peitsch MC (1996) ProMod and Swiss-Model: internet-based tools for automated comparative protein modelling. Biochem Soc Trans 24:274–279
Replius C (1983) Technological production of lactase and lactose-hydrolyzed milk. In: Delmont J (eds Milk intolerances and rejection. Karger, Basel, New York City pp 7–62
Rhaese HJ, Boetker NK (1973) The molecular basis of mutagenesis by methyl and ethyl methanesulfonates. Eur J Biochem 32:166–172
Rossi M, Ciaramella M, Cannio R, Pisani FM, Moracci M, Bartolucci S (2003) Extremophiles 2002. J Bacteriol 185:3683–3689
Russell NJ (2000) Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 4:83–90
Russell NJ, Gerike U, Danson MJ, Hough DW, Taylor GL (1998) Structural adaptations of the cold-active citrate synthase from an Antarctic bacterium. Structure 6:351–356
Sato S, Hutchinson III CA, Harris JL (1977) A thermostable sequence-specific endonuclease from Thermus aquaticus. Proc Natl Acad Sci USA 74:542–546
Sheridan PP, Panasik Jr. N, Coombs JM, Brenchley JE (2000) Approaches for deciphering the structural basis of low temperature enzyme activity. Biochim Biophys Acta 1543:417–433
Taguchi S, Orzaki A, Momose H (1998) Engineering of a cold-adapted protease by sequential random mutagenesis and screening system. Appl Environ Microbiol 64:492–495
Trimbur DE, Gutshall KR, Prema P, Brenchley JE (1994) Characterization of a psychrotrophic Arthrobacter gene and its cold-active β-galactosidase. Appl Environ Microbiol 60:4544–4552
Ulrich JT, McFeters GA, Temple KL (1972) Induction and characterization of β-galactosidase in an extreme thermophile. J Bacteriol 110:691–698
Wiehlman K, Braun R, Fitzpatrick J (1988) Investigation of the bicinchoninic acid protein assay: identification of the groups responsible for color formation. Anal Biochem 175:231–237
Wintrode PL, Miyazaki K, Arnold FH (2000) Cold-adaptation of a mesophilic subtilisin-like protease by laboratory evolution. J Biol Chem 275:31635–31640
Zartler ER, Jenney FE, Terrell M, Eidsness MK, Adams MWW, Prestegard JH (2001) Structural basis for thermostability in aporubredoxins from Pyrococcus furiosus and Clostridium pasterianum. Biochemistry 40:7279–7290
Zavodszky P, Kardos J, Svingor A, Petsko GA (1998) Adjustment of conformational flexibility is a key event in the thermal adaptation of proteins. Proc Natl Acad Sci USA 95:7406–7411
Acknowledgments
We thank members of our research group for helpful discussions and suggestions. We would also like to thank Kevin Gutshall for his help with the directed mutagenesis, and Al Phillips for discussions concerning the kinetics experiments and the manuscript. This work was supported by Department of Energy grant DE-FG02-93ER20117. James Coker was partially supported by NSF Research Training Grant DBI-9602232.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Wiegel
Rights and permissions
About this article
Cite this article
Coker, J.A., Brenchley, J.E. Protein engineering of a cold-active β-galactosidase from Arthrobacter sp. SB to increase lactose hydrolysis reveals new sites affecting low temperature activity. Extremophiles 10, 515–524 (2006). https://doi.org/10.1007/s00792-006-0526-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-006-0526-z