Abstract
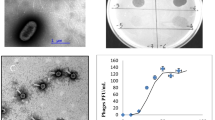
The surface sands of the Sahara Desert are exposed to extremes of ultraviolet light irradiation, desiccation and temperature variation. Nonetheless, the presence of bacteria has recently been demonstrated in this environment by cultivation methods and by 16S rDNA analyses from total DNA isolated from surface sands. To discern the presence of bacteriophages in this harsh environment, we searched for extracellular phages and intracellularly located phages present as prophages or within pseudolysogens. Mild sonication of the sand, in different liquid culture media, incubated with and without Mitomycin-C, was followed by differential centrifugation to enrich for dsDNA phages. The resulting preparations, examined by electron microscopy, revealed the presence of virus-like particles with a diversity of morphotypes representative of all three major double-stranded DNA bacteriophage families (Myoviridae, Siphoviridae and Podoviridae). Moreover, pulsed-field gel electrophoresis of DNA, extracted from the enriched bacteriophage preparations, revealed the presence of distinct bands suggesting the presence of putative dsDNA phage genomes ranging in size from 45 kb to 270 kb. Characterization of the bacteriophages present in the surface sands of the Sahara Desert extends the range of environments from which bacteriophages can be isolated, and provides an important point of departure for the study of phages in extreme terrestrial environments.




Similar content being viewed by others
References
Ackermann H, DuBow M (1987) Viruses of procaryotes. CRC Press, Boca Raton, Florida, USA
Alonso MC, Jimenez-Gomez F, Rodriguez J, Borrego JJ (2001) Distribution of virus-like particles in an oligotrophic marine environment (Alboran Sea, Western Mediterranean). Microb Ecol 42:407–415
Ashelford KE, Day MJ, Bailey MJ, Lilley AK, Fry JC (1999) In situ population dynamics of bacterial viruses in a terrestrial environment. Appl Environ Microbiol 65:169–174
Bergh O, Borsheim KY, Bratbak G, Heldal M (1989) High abundance of viruses found in aquatic environments. Nature 340:467–468
Breitbart M, Wegley L, Leeds S, Schoenfeld T, Rohwer F (2004) Phage community dynamics in hot springs. Appl Environ Microbiol 70:1633–1640
Canchaya C, Proux C, Fournous G, Bruttin A, Brussow H (2003) Prophage genomics. Microbiol Mol Biol Rev 67:238–276
Curtis TP, Sloan WT, Scannell JW (2002) Estimating prokaryotic diversity and its limits. Proc Natl Acad Sci USA 99:10494–10499
Davey ME, O’Toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867
Dobrindt U, Reidl J (2000) Pathogenicity islands and phage conversion: evolutionary aspects of bacterial pathogenesis. Int J Med Microbiol 290:519–527
Donlon RM (2002) Biofilms: microbial life on surfaces. Emerging Infect Diseases 8:881–890
Drexler H (1988) Bacteriophage T1. In: Calendar R (ed) The Bacteriophages. Plenum, New York, pp 235–258
Elasri MO, Miller RV (1999) Study of the response of a biofilm bacterial community to UV radiation. Appl Environ Microbiol 65:2025–2031
Elasri MO, Reid T, Hutchens S, Miller RV (2000) Response of a Pseudomonas aeruginosa biofilm community to DNA-damaging chemical agents. FEMS Microbiol Ecol 33:21–25
Forterre P (2001) New viruses for the new millennium. Trends Microbiol 9:114
Fuhrman JA (1999) Marine viruses and their biogeochemical and ecological effects. Nature 399:541–548
Fuhrman JA, Noble RT (1995) Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol Oceanorgr 40:1236–1242
Fuhrman JA, Schwalbach M (2003) Viral influence on aquatic bacterial communities. Biol Bull 204:192–195
Geslin C, Le Romancer M, Erauso G, Gaillard M, Perrot G, Prieur D (2003) PAV1, the first virus-like particle isolated from a hyperthermophilic euryarchaeote, “ Pyrococcus abyssi”. J Bacteriol 185:3888–3894
Heulin T, Barakat M, Christen R, Lesourd M, Sutra L, De Luca G, Achouak W (2003) Ramlibacter tataouinensis gen. nov., sp. nov., and Ramlibacter henchirensis sp. nov., cyst-producing bacteria isolated from subdesert soil in Tunisia. Int J Syst Evo Microbiol 53:589–594
Jiang SC, Paul JH (1998) Gene transfer by transduction in the marine environment. Appl Environ Microbiol 64:2780–2787
Lucchini S, Desiere F, Brussow H (1999) Similarly organized lysogeny modules in temperate Siphoviridae from low GC content gram-positive bacteria. Virology 263:427–435
Marie D, Brussaard CPD, Thyrhaug R, Bratbak G, Vaulot D (1999) Enumeration of marine viruses in culture and natural samples by flow cytometry. Appl Environ Microbiol 65:45–52
Paul J (2000) Ecology of bacteriophages in nature. In: Hurst C (ed) Viral ecology. Academic Press, San Diego, pp 211–246
Pedulla ML, Ford ME, Houtz JM, Karthikeyan T, Wadsworth C, Lewis JA, Jacobs-Sera D, Falbo J, Gross J, Pannunzio NR, Brucker W, Kumar V, Kandasamy J, Keenan L, Bardarov S, Kriakov J, Lawrence JG, Jacobs WR Jr, Hendrix RW, Hatfull GF (2003) Origins of highly mosaic mycobacteriophage genomes. Cell 113:171–182
Peng X, Blum H, She Q, Mallok S, Brugger K, Garrett RA, Zillig W, Prangishvili D (2001) Sequences and replication of genomes of the archaeal rudiviruses SIRV1 and SIRV2: relationships to the archaeal lipothrixvirus SIFV and some eukaryal viruses. Virology 291:226–234
Proctor LM, Fuhrman JA (1990) Viral mortality of marine bacteria and cyanobacteria. Nature 343:60–62
Rice G, Stedman K, Snyder J, Wiedenheft B, Willits D, Brumfield S, McDermott T, Young MJ (2001) Viruses from extreme thermal environments. Proc Natl Acad Sci USA 98:13341–13345
Ripp S, Miller RV (1998) Dynamics of the pseudolysogenic response in slowly growing cells of Pseudomonas aeruginosa. Microbiology 144 :2225–2232
Rondon MR, August PR, Bettermann AD, Brady SF, Grossman TH, Liles MR, Loiacono KA, Lynch BA, MacNeil IA, Minor C, Tiong CL, Gilman M, Osburne MS, Handelsman J, Goodman RM (2000) Cloning the soil metagenome: a strategy for accessing the genetic and functional diversity of uncultured microorganisms. Appl Environ Microbiol 66:2541–2547
Staley JT, Konopka A (1985) Measurement of in situ activities of non-photosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol 39:321–346
Weinbauer MG (2004) Ecology of prokaryotic viruses. FEMS Microbiol Rev 28:127–181
Williamson SJ, McLaughlin MR, Paul JH (2001) Interaction of the PhiHSIC virus with its host: lysogeny or pseudolysogeny?. Appl Environ Microbiol 67:1682–1688
Williamson KE, Wommack KE, Radosevich M (2003) Sampling natural viral communities from soil for culture-independent analyses. Appl Environ Microbiol 69:6628–6633
Wommack KE, Colwell RR (2000) Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64:69–114
Acknowledgements
The authors would like to thank Jeril Degrouard and Danielle Jaillard for their help with the electron microscopy, Suzanne Sommer and the members of her group for their generosity and aid, and the Referees for their insightful comments and criticisms. This work was supported by the GEOMEX program of the Centre National de la Recherche Scientifique (CNRS), France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Antranikian
Magali Prigent and Magali Leroy contributed equally to this work
Rights and permissions
About this article
Cite this article
Prigent, M., Leroy, M., Confalonieri, F. et al. A diversity of bacteriophage forms and genomes can be isolated from the surface sands of the Sahara Desert. Extremophiles 9, 289–296 (2005). https://doi.org/10.1007/s00792-005-0444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-005-0444-5