Abstract
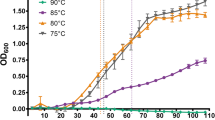
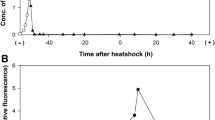
The thermal stress response of the hyperthermophilic bacterium Thermotoga maritima was characterized using a 407-open reading frame-targeted cDNA microarray. Transient gene expression was followed for 90 min, following a shift from 80°C to 90°C. While some aspects of mesophilic heat-shock response were conserved in T. maritima, genome content suggested differentiating features that were borne out by transcriptional analysis. Early induction of predicted heat-shock operons hrcA-grpE-dnaJ (TM0851-TM0850-TM0849), groES-groEL (TM0505-TM0506), and dnaK-sHSP (TM0373-TM0374) was consistent with conserved CIRCE elements upstream of hrcA and groES. Induction of the T. maritima rpoE/sigW and rpoD/sigA homologs suggests a mechanism for global heat-shock response in the absence of an identifiable ortholog to a major heat-shock sigma factor. In contrast to heat-shock response in Escherichia coli, the majority of genes encoding ATP-dependent proteases were downregulated, including clpP (TM0695), clpQ (TM0521), clpY (TM0522), lonA (TM1633), and lonB (TM1869). Notably, T. maritima showed indications of a late heat-shock response with the induction of a marR homolog (TM0816), several other putative transcriptional regulators (TM1023, TM1069), and two α-glucosidases (TM0434 and TM1068). Taken together, the results reported here indicate that, while T. maritima shares core elements of the bacterial heat-shock response with mesophiles, the thermal stress regulatory strategies of this organism differ significantly. However, it remains to be elucidated whether these differences are related to thermophilicity or phylogenetic placement.



Similar content being viewed by others
References
Aldsworth TG, Sharman RL, Dodd CER (1999) Bacterial suicide through stress. Cell Mol Life Sci 56:378–383
Ariza RR, Cohen SP, Bachhawat N, Levy SB, Demple B (1994) Repressor mutations in the marRAB operon that activate oxidative stress genes and multiple antibiotic resistance in Escherichia coli. J Bacteriol 176:143–148
Arnosti DN (1990) Regulation of Escherichia coli sigma F RNA polymerase by flhD and flhC flagellar regulatory genes. J Bacteriol 172:4106–4108
Britton RA, Eichenberger P, Gonzalez-Pastor JE, Fawcett P, Monson R, Losick R, Grossman AD (2002) Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J Bacteriol 184:4881–4890
Chhabra SR, Shockley KR, Conners SB, Scott KL, Wolfinger RD, Kelly RM (2003) Carbohydrate-induced differential gene expression patterns in the hyperthermophilic bacterium Thermotoga maritima. J Biol Chem 278:7540–7552
Cohen SP, Yan W, Levy SB (1993) A multidrug-resistance regulatory chromosomal locus is widespread among enteric bacteria. J Infect Dis 168:484–488
Dartigalongue C, Missiakas D, Raina S (2001) Characterization of the Escherichia coli sigma E regulon. J Biol Chem 276:20866–20875
Derre I, Rapoport G, Msadek T (1999) CtsR, a novel regulator of stress and heat-shock response, controls clp and molecular chaperone gene expression in Gram-positive bacteria. Mol Microbiol 31:117–131
Dickman MJ, Ingleston SM, Sedelnikova SE, Rafferty JB, Lloyd RG, Grasby JA, Hornby DP (2002) The RuvABC resolvasome. Eur J Biochem 269:5492–5501
Grandvalet C, Servant P, Mazodier P (1997) Disruption of hspR, the repressor gene of the dnaK operon in Streptomyces albus G. Mol Microbiol 23:77–84
Grossman AD, Erickson JW, Gross CA (1984) The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell 38:383–390
Helden J van, Andre B, Collado-Vides J (2000) A Web site for the computational analysis of yeast regulatory sequences. Yeast 16:177–187
Helmann JD, Chamberlin MJ (1987) DNA sequence analysis suggests that expression of flagellar and chemotaxis genes in Escherichia coli and Salmonella typhimurium is controlled by an alternative sigma factor. Proc Natl Acad Sci USA 84:6422–6424
Helmann JD, Wu MFW, Kobel PA, Gamo FJ, Wilson M, Morshedi MM, Navre M, Paddon C (2001) Global transcriptional response of Bacillus subtilis to heat shock. J Bacteriol 183:7318–7328
Hobbie JE, Daley RJ, Jasper S (1977) Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33:1225–1228
Homuth G, Masuda S, Mogk A, Kobayashi Y, Schumann W (1997) The dnaK operon of Bacillus subtilis is heptacistronic. J Bacteriol 179:1153–1164
Huber R, Langworthy TA, Konig H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique, extremely thermophilic eubacteria growing up to 90°C. Arch Microbiol 144:324–333
Kim DY, Kim DR, Ha SC, Lokanath NK, Lee CJ, Hwang HY, Kim KK (2003) Crystal structure of the protease domain of a heat-shock protein HtrA from Thermotoga maritima. J Biol Chem 278:6543–6551
Koonin EV, Aravind L, Galperin MY (2000) In: Storz G, Hengge-Aronis R (eds) Bacterial stress responses. ASM, Washington, D.C., pp 417–444
Kruger E, Hecker M (1998) The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat-shock genes. J Bacteriol 180:6681–6688
Lehnen D, Blumer C, Polen T, Wackwitz B, Wendisch VF, Unden G (2002) LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol Microbiol 45:521–532
Liu X, Matsumura P (1994) The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J Bacteriol 176:7345–7351
Lopez-Garcia P, Forterre P (2000). In: Storz G, Henggep-Aronis R (eds) Bacterial stress response. ASM, Washington, D.C., pp 369–382
Martin RG, Gillette WK, Rhee S, Rosner JL (1999) Structural requirements for marbox function in transcriptional activation of mar/sox/rob regulon promoters in Escherichia coli: sequence, orientation and spatial relationship to the core promoter. Mol Microbiol 34:431–441
Nelson KE, Clayton RA, Gill SR, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Nelson WC, Ketchum KA, McDonald L, Utterback TR, Malek JA, Linher KD, Garrett MM, Stewart AM, Cotton MD, Pratt MS, Phillips CA, Richardson D, Heidelberg J, Sutton GG, Fleischmann RD, Eisen JA, Fraser CM, et al (1999) Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima. Nature 399:323–329
Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG (2000) The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668
Pysz MA, Rinker KD, Shockley KR, Kelly RM (2001) In: Hyperthermophilic enzymes, part A, vol 330, pp 31–40
Raina S, Missiakas D, Georgopoulos C (1995) The rpoE gene encoding the sigma E (sigma 24) heat-shock sigma factor of Escherichia coli. EMBO J 14:1043–1055
Raivio TL, Silhavy TJ (2001) Periplasmic stress and ECF sigma factors. Annu Rev Microbiol 55:591–624
Richmond CS, Glasner JD, Mau R, Jin H, Blattner FR (1999) Genome-wide expression profiling in Escherichia coli K-12. Nucleic Acids Res 27:3821–3835
Rinker KD, Kelly RM (2000) Effect of carbon and nitrogen sources on growth dynamics and exopolysaccharide production for the hyperthermophilic archaeon Thermococcus litoralis and bacterium Thermotoga maritima. Biotechnol Bioeng 69:537–547
Rouviere PE, De Las Penas A, Mecsas J, Lu CZ, Rudd KE, Gross CA (1995) rpoE, the gene encoding the second heat-shock sigma factor, sigma E, in Escherichia coli. EMBO J 14:1032–1042
Schulz A, Schumann W (1996) hrcA, the first gene of the Bacillus subtilis dnaK operon encodes a negative regulator of class I heat-shock genes. J Bacteriol 178:1088–1093
Schumacher MA, Brennan RG (2002) Structural mechanisms of multidrug recognition and regulation by bacterial multidrug transcription factors. Mol Microbiol 45:885–893
Shockley KR, Ward DE, Chhabra SR, Conners SB, Montero CI, Kelly RM (2003) Heat-shock response by the hyperthermophilic archaeon Pyrococcus furiosus. Appl Environ Microbiol 69:2365–2371
Song HK, Bochtler M, Azim MK, Hartmann C, Huber R, Ramachandran R (2003) Isolation and characterization of the prokaryotic proteasome homolog HslVU (ClpQY) from Thermotoga maritima and the crystal structure of HslV. Biophys Chem 100:437–452
Stetter KO (1985) Extremely thermophile bacteria. Naturwissenschaften 72:291–300
Stintzi A (2003) Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J Bacteriol 185:2009–2016
Stohl EA, Brockman JP, Burkle KL, Morimatsu K, Kowalczykowski SC, Seifert HS (2003) Escherichia coli RecX inhibits RecA recombinase and coprotease activities in vitro and in vivo. J Biol Chem 278:2278–2285
Versteeg S, Escher A, Wende A, Wiegert T, Schumann W (2003) Regulation of the Bacillus subtilis heat-shock gene htpG is under positive control. J Bacteriol 185:466–474
Winterling KW, Levine AS, Yasbin RE, Woodgate R (1997) Characterization of DinR, the Bacillus subtilis SOS repressor. J Bacteriol 179:1698–1703
Winterling KW, Chafin D, Hayes JJ, Sun J, Levine AS, Yasbin RE, Woodgate R (1998) The Bacillus subtilis DinR binding site: redefinition of the consensus sequence. J Bacteriol 180:2201–2211
Yoshida A, Nakano Y, Yamashita Y, Oho T, Shibata Y, Ohishi M, Koga T (1999) A novel dnaK operon from Porphyromonas gingivalis. FEBS Lett 446:287–291
Yura T, Nakahigashi K (1999) Regulation of the heat-shock response. Curr Opin Microbiol 2:153–158
Yura T, Kanemori M, Morita T (2000). In: Storz G, Hennge-Aronis R (eds) Bacterial stress responses. ASM, Washington, D.C., pp 3–18
Zverlov VV, Schwarz WH (1999) Organization of the chromosomal region containing the genes lexA and topA in Thermotoga neapolitana. Primary structure of LexA reveals phylogenetic relevance. Syst Appl Microbiol 22:174–178
Acknowledgments
This work was supported in part by the Department of Energy (Energy Biosciences Program). S.B.C. acknowledges support from an NIEHS bioinformatics traineeship. M.R.J. acknowledges support from a GAANN fellowship. The authors wish to thank R. Wolfinger and K. Scott, SAS Institute, Cary, N.C., for help with implementing the mixed model analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.N. Reeve
Rights and permissions
About this article
Cite this article
Pysz, M.A., Ward, D.E., Shockley, K.R. et al. Transcriptional analysis of dynamic heat-shock response by the hyperthermophilic bacterium Thermotoga maritima . Extremophiles 8, 209–217 (2004). https://doi.org/10.1007/s00792-004-0379-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-004-0379-2