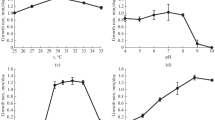
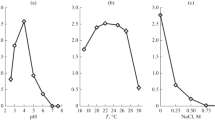
Abstract
The halophilic melanized yeast-like fungi Hortaea werneckii, Phaeotheca triangularis, and the halotolerant Aureobasidium pullulans, isolated from salterns as their natural environment, were grown at different NaCl concentrations and their membrane lipid composition and fluidity were examined. Among sterols, besides ergosterol, which was the predominant one, 23 additional sterols were identified. Their total content did not change consistently or significantly in response to raised NaCl concentrations in studied melanized fungi. The major phospholipid classes were phosphatidylcholine and phosphatidylethanolamine, followed by anionic phospholipids. The most abundant fatty acids in phospholipids contained C16 and C18 chain lengths with a high percentage of C18:2Δ9,12. Salt stress caused an increase in the fatty acid unsaturation in the halophilic H. werneckii and halotolerant A. pullulans but a slight decrease in halophilic P. triangularis. All the halophilic fungi maintained their sterol-to-phospholipid ratio at a significantly lower level than did the salt-sensitive Saccharomyces cerevisiae and halotolerant A. pullulans. Electron paramagnetic resonance (EPR) spectroscopy measurements showed that the membranes of all halophilic fungi were more fluid than those of the halotolerant A. pullulans and salt-sensitive S. cerevisiae, which is in good agreement with the lipid composition observed in this study.





Similar content being viewed by others
References
Andreishcheva EN, Isakova EP, Sidorov NN, Abramova NB, Ushakova NA, Shaposhnikov GL, Soares MIM, Zvyagilskaya RA (1999) Adaptation to salt stress in a salt-tolerant strain of the yeast Yarrowia lipolytica. Biochemistry (Moscow) 64:1061–1067
Demel RA, De Kruyff B (1976) The function of sterols in membranes. Biochim Biophys Acta 457:109–132
Gunde-Cimerman N, Zalar P, Hoog S de, Plemenitaš A (2000) Hypersaline waters in salterns: natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol 32:235–240
Hosono K (1992) Effect of salt stress on lipid composition and membrane fluidity of the salt-tolerant yeast Zygosaccharomyces rouxii. J Gen Microbiol 138:91–96
Hossack JA, Rose AH (1976) Fragility of plasma membranes in Saccharomyces cerevisiae enriched with different sterols. J Bacteriol 127:67–75
Kaneko H, Hosohara M, Tanaka M, Itoh T (1976) Lipid composition of 30 species of yeast. Lipids 11:837–844
Kates M (1986) Techniques of lipidology: isolation, analysis and identification of lipids, 2nd rev edn. Elsevier, Amsterdam
Khaware RK, Koul A, Prasad R (1995) High membrane fluidity is related to NaCl stress in Candida membranefaciens. Biochem Mol Biol Int 35:875–880
Lösel DM (1988) Fungal lipids. In: Ratledge C, Wilkinson SG (eds) Microbial lipids. Academic Press, London, pp 699–805
Lösel DM (1990) Lipids in the structure and function of fungal membranes. In: Kuhn PJ, Trinci APJ, Jung MJ, Goosey MW, Copping LG (eds) Biochemistry of the cell walls and membranes in fungi. Springer, Berlin Heidelberg New York, pp 119–133
Méjanelle L, Lopez JF, Gunde-Cimerman N, Grimalt JO (2000) Sterols of melanized fungi from hypersaline environments. Org Geochem 31:1031–1040
Mercer EI (1984) The biosynthesis of ergosterol. Pestic Sci 15:133–155
Parks LW (1978) Metabolism of sterols in yeast. CRC Crit Rev Microbiol 6:301–341
Quinn PJ (1981) The fluidity of cell membranes and its regulation. Prog Biophys Mol Biol 38:1–104
Russell NJ (1989) Adaptive modifications in membranes of halotolerant and halophilic microorganisms. J Bioenerg Biomembr 21:93–113
Russell NJ, Evans RI, Steeg PF ter, Hellemons J, Verheul A, Abee T (1995) Membranes as a target for stress adaptation. Int J Food Microbiol 28:255–261
Schara M, Pečar S, Svetek J (1990) Reactivity of hydrophobic nitroxides in lipid bilayers. Colloids Surf 45:303–312
Sharma SC, Raj D, Forouzandeh M, Bansal MP (1996) Salt-induced changes in lipid composition and ethanol tolerance in Saccharomyces cerevisiae. Appl Biochem Biotechnol 56:189–195
Štrancar J, Šentjurc M, Shara M (2000) Fast and accurate characterization of biological membranes by EPR spectral simulations of nitroxides. J Magn Reson 142:254–265
Tunblad-Johansson I, Andre L, Adler L (1987) The sterol and phospholipid composition of the salt-tolerant yeast Debaryomyces hansenii grown at various concentrations of NaCl. Biochim Biophys Acta 921:116–123
Weete JD (1989) Structure and function of sterols in fungi. Adv Lipid Res 23:115–167
Weete JD, Gandhi SR (1996) Biochemistry and molecular biology of fungal sterols. In: Brambl R, Marzluf GA (eds) Biochemistry and molecular biology. Springer, Berlin Heidelberg New York, pp 421–438
Zalar P, Hoog GS de, Gunde-Cimerman N (1999a) Ecology of halotolerant dothideaceous black yeasts. Stud Mycol 43:38–48
Zalar P, Hoog GS de, Gunde-Cimerman N (1999b) Taxonomy of the endoconidial black yeast genera Phaeotheca and Hyphospora. Stud Mycol 43:49–56
Zhang JY, Yu QT, Liu BN, Huang YH (1988) Chemical modification in mass spectrometry. VI. 2-alkenyl-4,4-dimethyloxazolines as derivatives for the double bond location of long-chain olefinic acids. Biomed Environ Mass Spectrom 15:33–44
Acknowledgements
Many thanks are due to Novo Nordisk, Denmark for the generous donation of a Glucanex lytic solution. We would also like to thank all our colleagues for their useful suggestions and technical assistance. M. Turk was supported by a fellowship from the Slovene Ministry of Education, Science and Sport.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W.D. Grant
Rights and permissions
About this article
Cite this article
Turk, M., Méjanelle, L., Šentjurc, M. et al. Salt-induced changes in lipid composition and membrane fluidity of halophilic yeast-like melanized fungi. Extremophiles 8, 53–61 (2004). https://doi.org/10.1007/s00792-003-0360-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-003-0360-5