Abstract
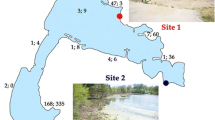
The virioplankton community structure along a salinity gradient from near seawater (40‰) to saturated sodium chloride brine (370‰) in a solar saltern was investigated by pulsed-field gel electrophoresis. Viral populations with genome sizes varying from 10 kb to 533 kb were detected. The viral community structure changed along the salinity gradient. Cluster analysis of the viral genome-banding pattern resulted in two main clusters. The virioplankton diversity within the samples with salinity from 40‰ to 150‰ was on the same cluster of a cladogram. The other group consisted of virioplankton from samples with salinity above 220‰. The virioplankton diversity in the different samples was calculated using the Shannon index. The diversity index demonstrated an increase in diversity in the samples along the gradient from 40‰ to 150‰ salinity, followed by a decrease in the diversity index along the rest of the salinity gradient. These results demonstrate how viral diversity changes from habitats that are considered one of the most common (seawater) to habitats that are extreme in salt concentrations (saturated sodium brine). The diversity index was highest in the environments that lie in between the most extreme and the most common.



Similar content being viewed by others
References
Antón J, Llobet-Brossa E, Rodríguez-Valera F, Amann R (1999) Fluorescence in situ hybridization of the prokaryotic community inhabiting crystallizer ponds. Environ Microbiol 1:517–523
Antón J, Rosselló-Mora R, Rodríguez-Valera F, Amann R (2000) Extremely halophilic Bacteria in crystallizer ponds from solar salterns. Appl Environ Microbiol 66:3052–3057
Benlloch S, Martínez-Murcia AJ, Rodríguez-Valera F (1995) Sequencing of bacterial and archaeal 16S rRNA genes directly amplified from a hypersaline environment. Syst Appl Microbiol 18:574–581
Benlloch S, Acinas SG, Martínez-Murcia AJ, Rodríguez-Valera F (1996) Description of prokaryotic biodiversity along the salinity gradient of a multipond solar saltern by direct PCR amplification of 16S rDNA. Hydrobiologia 329:19–31
Casamayor EO, Calderón-Paz JI, Pedrós-Alió C (2000) 5S rRNA fingerprints of marine bacteria, halophilic Archaea and natural prokaryotic assemblages along a salinity gradient. FEMS Microbiol Ecol 34:113–119
Castberg T, Larsen A, Sandaa R-A, Brussaard CPD, Egge J, Heldal M, Thyrhaug R, van Hannen EJ, Bratbak G (2001) Microbial population dynamics and diversity during blooms of the marine coccolithophorid Emiliania huxleyi (Haptophyta). Mar Ecol Prog Ser 221:39–46
Diez B, Antón J, Guixa- Boixareu N, Pedrós-Alió C, Rodríguez-Valera F (2000) Pulsed-field gel electrophoresis analysis of virus assemblages present in a hypersaline environment. Internal Microbiol 3:159–164
Guixa-Boixareu N, Calderón-Paz JI, Heldal M, Bratbak G, Pedrós-Alió C (1996) Viral lysis and bacterivory as prokaryotic loss factors along a salinity gradient. Aquat Microb Ecol 11:215–227
Javor BJ (1983) Plantonic standing crop and nutrients in a saltern ecosystem. Limnol Oceanogr 28:153–159
Larsen A, Castberg T, Sandaa R-A, Brussaard CPD, Egge J, Heldal M, Paulino A, Thyrhaug R, van Hannen EJ, Bratbak G (2001) Population dynamics and diversity of phytoplankton, bacteria and virus in a seawater enclosure. Mar Ecol Prog Ser 221:47–57
Oren A (1994) The ecology of the extremely halophilic Archaea. FEMS Microbiol Rev 13:415–440
Oren A (2002) Molecular ecology of extremely halophilic Archaea and Bacteria. FEMS Microbiol Ecol 39:1–7
Oren A, Rodríguez-Valera F (2001) The contribution of halophilic Bacteria to the red coloration of saltern crystallizer ponds. FEMS Microbiol Ecol 36:123–130
Oren A, Bratbak G, Heldal M (1997) Occurrence of virus-like particles in the Dead Sea. Extremophiles 1:143–149
Øvreås L, Bourne D, Sandaa R-A, Casamayor EO, Benlloch S, Goddard V, Smeardon G, Heldal M, Thingstad FT (2003) Response of bacterial and viral communities to nutrient manipulations in seawater mesocosms. Aquat Microb Ecol (in press)
Pedrós-Alió C, Calderón-Paz JI, MacLean MH, Medina G, Marrasé C, Gasol JM, Guixa- Boixareu N (2000) The microbial food web along salinity gradients. FEMS Microbiol Ecol 32:143–155
Rodríguez-Valera F (1988) Characteristics and microbial ecology of hypersaline environments. In: Rodríguez-Valera F (ed) Halophilic bacteria, vol I. CRC Press, Boca Raton, pp 3–30
Rodríguez-Valera F, Ruiz-Berraquero F, Ramos-Cormenzana A (1981) Characteristics of the heterotrophic bacterial populations in hypersaline environments of different salt concentrations. Microbiol Ecol 7:235–243
Rodríguez-Valera F, Ventosa A, Juez G, Imhoff JF (1985) Variation of environmental features and microbial populations with salt concentrations in a multi-pond saltern. Microbiol Ecol 11:107–115
Rodríguez-Valera F, Acinas SG, Antón J (1999) Contribution of molecular techniques to the study of microbial diversity in hypersaline environments. In: Oren A (ed) Microbiology and biochemistry of hypersaline environments. CRC Press, Boca Raton, pp 27–38
Shannon CE (1948) A mathematical theory of communication. Bell Syst Technol 27:379–423
Tang S-L, Nuttall S, Ngui K, Fisher C, Lopez P, Dyall-Smith M (2002) HF2 a double-stranded DNA tailed haloarchaeal virus with a mosaic genome. Mol Microbiol 44:283–296
Wommack KE, Ravel J, Hill RT, Chun J, Colwell RR (1999) Population dynamics of Chesapeake bay virioplankton: Total community analysis by pulsed-field gel electrophoresis. Appl Environ Microbiol 65:231–240
Acknowledgments
Øivind Enger provided valuable and useful comments on the manuscript. Svein Norland is thanked for providing the software used for statistical analysis and for discussions on the interpretation of the results. This work was financed by the EC through contract MAS3-CT97-0154 "MIDAS" and from The Research Council of Norway (project no. 113037/120 and 121425/420).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W.D. Grant
Rights and permissions
About this article
Cite this article
Sandaa, RA., Foss Skjoldal, E. & Bratbak, G. Virioplankton community structure along a salinity gradient in a solar saltern. Extremophiles 7, 347–351 (2003). https://doi.org/10.1007/s00792-003-0328-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-003-0328-5