Abstract
Autism spectrum disorder (ASD) is a disabling neurodevelopmental condition with complex etiology. Emerging evidence has pointed to maternal atopy as a possible risk factor. It is hypothesized that maternal atopic disease during pregnancy can lead to increased levels of inflammatory cytokines in fetal circulation via placental transfer or increased production. These cytokines can then pass through the immature blood–brain barrier, causing aberrant neurodevelopment via mechanisms including premature microglial activation. The objective of this study is to systematically review observational studies that investigate whether a maternal history of atopic disease (asthma, allergy, or eczema/atopic dermatitis) is associated with a diagnosis of ASD in offspring. A search was conducted in Ovid MEDLINE, PsycINFO, and Embase databases for relevant articles up to November 2021; this was later updated in January 2022. Observational studies published in peer-reviewed journals were included. Data were synthesized and qualitatively analyzed according to the specific atopic condition. Quality assessment was done using the Newcastle–Ottawa Scale. Nine articles were identified, with all including asthma as an exposure, alongside four each for allergy and eczema. Findings were inconsistent regarding the association between a maternal diagnosis of either asthma, allergy, or eczema, and ASD in offspring, with variations in methodology contributing to the inconclusiveness. More consistent associations were demonstrated regarding maternal asthma that was treated or diagnosed during pregnancy. Evidence suggests that symptomatic maternal asthma during pregnancy could be associated with ASD in offspring, underscoring the importance of effective management of atopic conditions during pregnancy. Further research is needed, particularly longitudinal studies that use gold-standard assessment tools and correlate clinical outcomes with laboratory and treatment data.
PROSPERO Registration Number and Date: CRD42018116656, 26.11.2018.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition distinguished by early-onset deficits in social communication, as well as restricted, repetitive behaviors and interests [1, 2]. This disorder may cause significant impairment in daily functioning, and is associated with high levels of comorbidity with other medical, developmental, and psychiatric conditions [3]. Prevalence rates have risen over the past two decades, with the median worldwide prevalence estimated at 0.62–0.7% [4, 5]. Despite great strides in awareness and understanding, much is still not known regarding the etiology of ASD. Current evidence points to autism as resulting from the interaction of genetic factors with the environment, both prenatal and postnatal [6, 7].
Recent studies propose inflammation as a notable risk factor [8], with specific attention to maternal immune dysregulation during pregnancy potentially affecting vulnerable stages of fetal brain development [9, 10]. Pre-clinical studies have demonstrated that maternal immune activation in mice and rhesus monkeys led to increased autism-like behaviors in offspring [11, 12]. Recent reviews and meta-analyses have also concluded that ASD in offspring is associated with both maternal autoimmune disease [13] and maternal infection [14, 15] which further indicates the potential role of maternal immune activation (MIA) on fetal neurodevelopment.
Emerging evidence has also pointed to maternal atopy, a distinct form of immune dysregulation, as a risk factor. In 2005, a case–control study initially reported an association between a diagnosis of maternal asthma and allergy with autism in offspring [16], setting the stage for other recent studies to investigate the potential relationship [15, 17, 18]. It is hypothesized that maternal atopic disease during pregnancy can lead to increased levels of inflammatory cytokines in fetal circulation, either through placental transfer or increased production. These cytokines can then pass through the immature blood–brain barrier, causing premature microglial activation and consequently, aberrant neurodevelopment [9, 19, 20].
In line with the above hypothesis regarding atopy, a recent cohort study concludes that the maternal prenatal IgE, an immunoglobulin with an essential role in atopic reactions, may influence neurodevelopment of the offspring [21, 22].
Despite the links between MIA and ASD pathophysiology, some important concerns how MIA impacts the developing fetal brain at genomic and epigenomic levels need still to be clarified [23]. Autism Spectrum Disorder and other neurodevelopmental diseases in humans are caused by the vulnerability of developing brain to epigenetic modifications, which are assumed to be the genome's adaptation to a changing environment [24,25,26]. Research studies suggest that epigenetic mechanisms may play a role in the relationship between environmental factors and immune system changes [27]. The changes in the expression and methylation of immune system-related genes in ASD could happen in response to environmental factors such as MIA [24].
Some studies on animal models reveal that offspring's long-term behavioral abnormalities after exposure to prenatal maternal allergic asthma (MAA) are due to epigenetic modification within the brain cells, via DNA methylation and histone acetylation [24, 28, 29]. Vogel et al. performed first whole genome-wide analyses of the DNA methylome and transcriptome of microglia isolated from juvenile offspring of MAA dams (mother of an animal) and their findings suggest an overlap between MAA and ASD-relevant epigenetic changes in microglia [24]. Another study by Lombardo et al. reported a potential relationship between ASD risk and dysregulation in cerebral gene expression in fetus as a result of MIA [23].
Another recent Swedish population case–control study found association between maternal asthma and increased risk of offspring ASD [17]. Although there are reports of mixed evidence on the associations between maternal asthma during pregnancy and child cognitive and behavioral development [30], one of the most recent systematic reviews [15] found that exposure to maternal asthma in the first and second trimester is associated with childhood ASD, which has consolidated the evidence regarding MIA as one of the pathways causing fetal neuroinflammation.
It is hoped that the current review will contribute to fill the knowledge gap in the understanding of inflammation-related prenatal risk factors for ASD and complement the existing reviews which investigate atopic diseases in children and their risk for autism [31,32,33].
Potentially, this review also has clinically relevant implications in terms of treatment and management of maternal atopic conditions towards healthy outcomes in neurodevelopmental disorders [34].
Therefore, the objective of this study is to systematically review cross-sectional, case–control, and cohort studies that investigate whether a maternal history of atopic disease (asthma, allergy, or eczema/atopic dermatitis) is associated with a diagnosis of autism spectrum disorder in offspring.
Methodology
Literature search
This systematic review was conducted according to the recommendations of the Preferred Reporting Items for Systematic Reviews (PRISMA) statement [35] and registered with PROSPERO (CRD42018116656).
A search was conducted in Ovid MEDLINE, PsycINFO, and Embase databases for articles up to January 2022, without restrictions regarding language, date, or article type. Keywords used were maternal OR prenatal OR pregnancy OR famil* AND atopy OR asthma OR allerg* OR eczema OR atopic dermatitis AND autis* OR Asperger OR ASD.
Study selection
Titles and abstracts of articles were screened to exclude discernibly irrelevant studies, after which the full text of the remaining studies were assessed according to inclusion criteria. In addition, the reference lists of the included articles were examined, and further articles were identified this way to be included in the review.
Observational studies that investigated the association between maternal history of atopic disease (asthma, allergy, or eczema) and autism spectrum disorder in offspring were included. Studies were excluded if they were case reports, case series, or animal studies. Studies that were not published in peer-reviewed journals were likewise excluded.
Data extraction and synthesis
The following data were obtained from each study: author, publication year, country of origin, type of study, number of subjects, age, method of assessment of atopy and autism, and relevant study outcomes. Data were synthesized and qualitatively analyzed according to the specific atopic condition.
Quality assessment
The Newcastle–Ottawa Scale was used to assess the risk of bias, as it was specifically developed to provide a useful means to assess the quality of non-randomized studies in systematic reviews [36]. In the scale, stars are awarded for quality characteristics grouped according to three categories: selection of study groups, comparability of study groups, and ascertaining outcome/exposure (for cohort and case–control studies, respectively), for which a total of nine stars may be awarded to indicate high quality.
Methodology involved multiple reviewers for the systematic search and data extraction, consensus was achieved through discussion where there were initial disagreements.
Results
Study selection
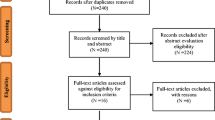
The initial database search yielded 524 citations, with 122 from MEDLINE, 78 from PsycINFO, and 324 from Embase. An additional two articles were identified by reviewing reference lists. After removing duplicates, 421 studies were screened initially by title, yielding 42 studies, and then by abstract, yielding 22 studies. Of the studies which qualified for full-text screening, nine met the full inclusion criteria (see Fig. 1).
Study characteristics are summarized in Tables 1, 2 and 3. All of the nine studies included maternal asthma as an exposure variable, while four studies each included maternal allergy and eczema. All of the studies were conducted in high-income countries [37]: four in the USA, three in Australia, and one each in the UK and Denmark. Eight were case–control studies and one was a cohort study. Quality assessment using the Newcastle–Ottawa Scale is summarized in Table 4.
Asthma
Contradictory findings were noted regarding the association between maternal asthma and autism in offspring (see Table 1) Three of the nine studies demonstrated a significant association, with odds ratios of 1.14 and 1.6, and the cohort study concluding that maternal asthma and allergies are linked with more severe social impairments in the offspring as measured by the Social Responsiveness Scale [16, 18, 38]. Meanwhile, two additional studies showed elevated associations, which, however, became non-significant once adjustment for sociodemographic factors was done [39, 40]. The remaining four studies found evidence of no significant association between the two variables [41,42,43,44].
The association between maternal asthma and offspring autism was more consistently significant if it was either treated [38, 40] or recorded during pregnancy, particularly in the first and second trimesters [16], with odds ratios ranging from 1.29 to 2.8. However, this latter finding was not replicated in the secondary analysis of another study [41].
To assess for autism, three of the more recent studies utilized gold-standard assessment tools such as the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview—Revised (ADI-R), with two of them finding no significant association [40, 41], and one a significant association [18]. The remaining studies ascertained the presence of a diagnosis of autism from medical records; therefore, it is notable that diagnosis in these cases was established via clinical assessment utilizing a method popular in the study location at the time.
Regarding the assessment of maternal asthma, five studies used medical records [16, 38, 39, 43, 44] and two used self-report measures [18, 42]. Two studies categorized mothers as having asthma if the condition was noted in either the medical record or a self-report measure [40, 41].
Allergy
Regarding maternal allergy and autism, two studies reported positive association [16, 18], with one reporting a notably high odds ratio of 2.5 for allergies diagnosed in the second trimester [16], whereas two studies found no evidence for an association [40, 41] (see Table 2).
Variation was noted in how exposures were assessed: by self-report [18], through maternal records [16], or either[40, 41]. Moreover, studies had varying definitions of what constituted an allergy in terms of exposures and manifestations, for instance two studies categorized eczema as a subtype of allergy [16, 41].
Eczema
For the relationship between maternal eczema and autism, two studies reported positive association [16, 41], with odds ratios at 1.8 and 1.4, respectively. Meanwhile another study found no evidence for association [42] (see Table 3). Studies again varied as to how exposures were assessed: by self-report [42], through maternal records [16], or either [40, 41]. Discrepancies were noted regarding classification of eczema within atopic and alongside other conditions; for example, eczema was considered as a subtype of allergy in one study [16], while another grouped eczema and psoriasis (an autoimmune condition) together [40].
Discussion
Summary of findings
In summary, mixed evidence was found regarding the association of a maternal asthma, allergies, or eczema diagnosis with autism in offspring. More consistent associations were demonstrated for asthma that was treated or diagnosed during pregnancy [16, 38, 40], which supports the hypothesis of maternal immune activation. Although most research has focused on autoimmune and infectious triggers, it has been proposed that other conditions such as maternal stress, environmental pollutants, as well as allergies and asthma can also contribute to the risk for ASD by activating maternal immune response [9, 20]. Recent preclinical experiments have demonstrated that a maternal asthma-allergy model is causative of autism-related behaviors in mouse offspring [45, 46]; and cytokines, particularly interleukin (IL)-6, have been proposed to be key mediators of this relationship [47] (see Fig. 2).
Asthma, like many other inflammatory diseases, increases interleukin (IL)-6 levels [48], which is the only proinflammatory cytokine known to pass from maternal to fetal circulation via the placenta [19, 49, 50]. Alternatively, the placenta itself can cause overproduction of IL-6 and other proinflammatory cytokines, a finding demonstrated in preclinical studies and human female offspring of mothers with maternal asthma [19, 51, 52]. Regardless of source, the cytokine imbalance in fetal circulation can breach the immature blood–brain barrier and prime previously quiescent microglia, the resident immune cells of the CNS. Microglia can then transform into a persistently amoeboid morphology, which are both actively phagocytic and capable of producing more cytokines and chemokines, thus causing the neuroinflammatory and neuro-morphological changes seen in ASD [8, 19]. Such a hypothesis is further supported by studies demonstrating increased cytokine levels in midgestational serum of mothers of offspring with ASD [53], as well as in amniotic fluid [54], and newborn blood spots of children eventually diagnosed with ASD [55], although levels of specific cytokines vary in each experiment.
Proinflammatory cytokines can also influence the fetal hypothalamic–pituitary–adrenal (HPA) axis. Whether maternal, placental, or fetal in origin, cytokines can stimulate release of corticotropin releasing hormone and arginine vasopressin from the fetal hypothalamus leading to excess fetal glucocorticoids, which can also affect fetal neurodevelopment and cause long-term changes in function of HPA axis [19, 56,57,58,59,60].
Besides the inhibitory effects of glucocorticoids in fetal neurodevelopment, the use of beta-2adrenergic agonists during pregnancy is found to correlate with greater risk of autism in offspring, independent of maternal asthma [61].
Epigenetic factors have been suggested as a possible mechanism linking maternal allergic asthma and ASD in offspring according to the recent reviews [25, 62].
One of the recent molecular studies also highlights MIA as a well-known risk factor for ASD; it postulates that MIA plays a role at very early stages of fetal neurodevelopment through causing dysregulation of the fetal brain transcriptome and downregulating expression of genes known to be important for ASD pathophysiology [24]. The first genome-wide analyses also point to maternal allergic asthma as a risk factor for ASD through pathogenic pathways that involve modifications in DNA methylation and transcription in microglia isolated from juvenile offspring thus, which impacts on microglial activity which is suggested to be a potential therapeutic target [24].
Maternal inflammation during pregnancy seems to play an important role in immune activation through the placenta and immature blood–brain barrier predisposing the offspring to be susceptible to future hits through microglial activation and modification of fetal epigenetic machinery [25, 62].
Chronic inflammation resulting from an atopic condition could then result in the neuroinflammatory processes described above, resulting in autism [32].
Study limitations
Some limitations of the studies may have contributed to the inconsistencies in findings. First, regarding the definition of autism, the use of clinical diagnosis to define autism has been subject to questions regarding interrater reliability [63]. Only recently have validated and reliable assessment tools such as the ADOS and ADI-R been used [64], notably accounting for only a third of the studies [18, 40, 41]. Second, the studies included in this review varied in terms of how atopic conditions were defined, diagnosed, and classified. Studies that extracted maternal atopy from medical records were heterogeneous in terms of timeframe, that is whether the diagnosis referred to a lifetime history of asthma, a new diagnosis during pregnancy, or an exacerbation of previously diagnosed asthma during the prenatal period [16, 38, 40, 41, 43, 44]. Variations in the type and classification of allergies and atopic dermatitis also make it challenging to compare studies side-by-side [16, 40, 41]. Third, studies that used self-report to define cases of atopy [18, 40,41,42] may have been affected by recall bias as parents of children with severe childhood diseases have been known to overreport possible risk factors in an effort to understand the etiology of their child’s condition [65]. Also, none of the studies were adjusted for a family history of autism, to account for genetic liability as a risk factor. Finally, all but one of the studies used a case–control design, which does not allow for a prospective follow-up of maternal asthma and child development, and therefore makes it challenging to presume causality.
Review limitations
Several limitations of this review must be considered. First, while a conscious decision was made to include articles only published in peer-reviewed journals to ensure quality of the research, some studies affected by publication bias, may have been potentially excluded. Moreover, all the included studies were conducted in high-income countries, possibly limiting the applicability of the results to low- and middle-income countries. Due to high heterogeneity and the inconsistencies between the reported findings in the included studies, a meta-analysis was not conducted; however, applying a meta-analysis following systematic reviews where appropriate would make valuable contributions to the scientific literature. Finally, the Newcastle–Ottawa Scale, the tool used for the risk-of-bias assessment, still has no definitive quantitative norms as to what score constitutes a high- or low-quality study [36]. Therefore, caution must be exercised in using the scores as a measure of study quality.
Conclusion and recommendations
In conclusion, this review found inconsistent evidence to determine that a diagnosis of asthma, allergies, or eczema is associated with autism in offspring, while there was more consistent evidence for asthma diagnosed or treated during the pregnancy. Such findings, if replicated by differently designed future studies, underscore, both to mothers-to-be, as well as family and professionals caring for them, the importance of effectively managing atopic maternal conditions during the prenatal period as a means to ensure healthy neurodevelopment. More longitudinal studies are likewise recommended for future research, particularly those that utilize gold-standard assessment tools for autism, clearly define the timing of atopic conditions, and correlate exposures with diagnostic (both laboratory and neuroimaging) and treatment data.
References
Organization WH. ICD-10: the ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. ICD-10: the ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research1993
Association AP (2013) Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub
Lai MC, Lombardo MV, Baron-Cohen S (2014) Autism. Lancet (London) 383(9920):896–910
Fombonne E (2009) Epidemiology of pervasive developmental disorders. Pediatr Res 65(6):591–598. https://doi.org/10.1203/PDR.0b013e31819e7203
Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C et al (2012) Global prevalence of autism and other pervasive developmental disorders. Autism Res 5(3):160–179
Ornoy A, Weinstein-Fudim L, Ergaz Z (2015) Prenatal factors associated with autism spectrum disorder (ASD). Reproduct Toxicol (Elmsford) 56:155–169
Herbert MRJ (2010) Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol 23(2):103–110
Siniscalco D, Schultz S, Brigida A, Antonucci NJP (2018) Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals 11(2):56
Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK (2018) Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol 299(Pt A):241–245
Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA et al (2014) Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 10(11):643
Malkova NV, Collin ZY, Hsiao EY, Moore MJ, Patterson PHJB (2012) Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immunity 26(4):607–616
Bauman MD, Iosif A-M, Smith SE, Bregere C, Amaral DG, Patterson PHJBP (2014) Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry 75(4):332–341
Chen SW, Zhong XS, Jiang LN, Zheng XY, Xiong YQ, Ma SJ et al (2016) Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: a systematic review and meta-analysis. Behav Brain Res 296:61–69
Jiang HY, Xu LL, Shao L, Xia RM, Yu ZH, Ling ZX et al (2016) Maternal infection during pregnancy and risk of autism spectrum disorders: a systematic review and meta-analysis. Brain Behav Immun 58:165–172
Han VX et al (2021) Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry 11(1):71
Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J (2005) Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case–control study. Arch Pediatrics Adolescent Med 159(2):151–157
Gong T et al (2019) Parental asthma and risk of autism spectrum disorder in offspring: a population and family-based case–control study. Clin Exp Allergy 49(6):883–891
Patel S et al (2020) Maternal immune conditions are increased in males with autism spectrum disorders and are associated with behavioural and emotional but not cognitive co-morbidiity. Transl Psychiatry 10(1):286
Ratnayake U, Quinn T, Walker D, Dickinson HJF (2013) Cytokines and the neurodevelopmental basis of mental illness. Front Neurosci 7:180
Estes ML, McAllister AK (2016) Maternal immune activation: implications for neuropsychiatric disorders. Science (New York) 353(6301):772–777
Straughen JK et al (2021) Prenatal IgE as a risk factor for the development of childhood neurodevelopmental disorders. Front Pediatr 14(9):601092
Justiz Vaillant AA, Modi P, Jan A (Jan 2022) Atopy. 2022 July 8. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing
Lombardo MV, Moon HM, Su J, Palmer TD, Courchesne E, Pramparo T (2018) Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol Psychiatry 23(4):1001–1013. https://doi.org/10.1038/mp.2017.15
Vogel Ciernia A, Careaga M, LaSalle JM, Ashwood P (2018) Microglia from offspring of dams with allergic asthma exhibit epigenomic alterations in genes dysregulated in autism. Glia 66(3):505–521. https://doi.org/10.1002/glia.23261
Han VX, Patel S, Jones HF, Nielsen TC, Mohammad SS, Hofer MJ, Gold W, Brilot F, Lain SJ, Nassar N, Dale RC (2021) Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry 11(1):71. https://doi.org/10.1038/s41398-021-01198-w
Vogel Ciernia A, LaSalle J (2016) The landscape of DNA methylation amid a perfect storm of autism aetiologies. Nat Rev Neurosci 17(7):411–423. https://doi.org/10.1038/nrn.2016.41
Nardone S, Elliott E (2016) The interaction between the immune system and epigenetics in the etiology of autism spectrum disorders. Front Neurosci 12(10):329. https://doi.org/10.3389/fnins.2016.00329
Basil P, Li Q, Dempster EL, Mill J, Sham PC, Wong CC, McAlonan GM (2014) Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry 4(9):e434. https://doi.org/10.1038/tp.2014.80
Richetto J, Massart R, Weber-Stadlbauer U, Szyf M, Riva MA, Meyer U (2017) Genome-wide DNA methylation changes in a mouse model of infection-mediated neurodevelopmental disorders. Biol Psychiatry 81(3):265–276. https://doi.org/10.1016/j.biopsych.2016.08.010
Whalen OM, Karayanidis F, Murphy VE, Lane AE, Mallise CA, Campbell LE (2019) The effects of maternal asthma during pregnancy on child cognitive and behavioral development: a systematic review. J Asthma 2:130–141
Billeci L, Tonacci A, Tartarisco G, Ruta L, Pioggia G, Gangemi SJA (2015) Association between atopic dermatitis and autism spectrum disorders: a systematic review. Am J Clin Dermatol 16(5):371–388
Tonacci A, Billeci L, Ruta L, Tartarisco G, Pioggia G, Gangemi S (2017) A systematic review of the association between allergic asthma and autism. Minerva Pediatr 69(6):538–550
Zheng Z, Zhang L, Zhu T, Huang J, Qu Y, Mu DJP (2016) Association between asthma and autism spectrum disorder: a meta-analysis. PLoS ONE 11(6):e0156662
Hafizi S et al (2019) Review of clinical studies targeting inflammatory pathways for individuals with autism. Front Psychiatry 10:849
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin Res Ed) 339:b2535
Hospital O. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://ohri.ca/programs/clinical_epidemiology/oxford.asp
The World Bank Group (2022) The World Bank website, 27.10,2022. <data.worldbank.org/country/XD>
Hisle-Gorman E, Susi A, Stokes T, Gorman G, Erdie-Lalena C, Nylund CM (2018) Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr Res 84(2):190–198
Langridge AT, Glasson EJ, Nassar N, Jacoby P, Pennell C, Hagan R et al (2013) Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS ONE (Electron Resour) 8(1):e50963
Croen LA et al (2019) Family history of immune conditions and autism spectrum and developmental disorders: findings from the study to explore early development. Autism Res 12(1):123–135
Lyall K, Ashwood P, Van de Water J, Hertz-Picciotto I (2014) Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disorders 44(7):1546–1555
Micali N, Chakrabarti S, Fombonne E (2004) The broad autism phenotype: findings from an epidemiological survey. Autism 8(1):21–37
Mouridsen SE, Rich B, Isager T, Nedergaard NJ (2007) Autoimmune diseases in parents of children with infantile autism: a case–control study. Dev Med Child Neurol 49(6):429–432
Leonard H, de Klerk N, Bourke J, Bower C (2006) Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann Epidemiol 16(6):448
Schwartzer J, Careaga M, Chang C, Onore C, Ashwood PJT (2015) Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Transl Psychiatry 5(4):e543
Schwartzer JJ, Careaga M, Coburn MA, Rose DR, Hughes HK, Ashwood P (2017) Behavioral impact of maternal allergic-asthma in two genetically distinct mouse strains. Brain Behav Immun 63:99–107
Parker-Athill EC, Tan JJN (2010) Maternal immune activation and autism spectrum disorder: interleukin-6 signaling as a key mechanistic pathway. Neurosignals 18(2):113–128
Rincon M, Irvin CGJI (2012) Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci 8(9):1281
Zaretsky MV, Alexander JM, Byrd W, Bawdon REJO (2004) Gynecology. Transfer of inflammatory cytokines across the placenta. Obstetrics Gynecol 103(3):546–550
Dahlgren J, Samuelsson A-M, Jansson T, Holmäng AJP (2006) Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatric Res 60(2):147
Scott NM, Hodyl NA, Murphy VE, Osei-Kumah A, Wyper H, Hodgson DM et al (2009) Placental cytokine expression covaries with maternal asthma severity and fetal sex. J Immunol 182(3):1411–1420
Hsiao EY, Patterson PHJB (2011) Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immunity 25(4):604–615
Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R et al (2011) Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: a case–control study. Mol Autism 2(1):13
Abdallah MW, Larsen N, Grove J, Nørgaard-Pedersen B, Thorsen P, Mortensen EL et al (2013) Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J Biol Psychiatry 14(7):528–538
Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I et al (2017) Neonatal cytokine profiles associated with autism spectrum disorder. Biol Psychiatry 81(5):442–451
Dunn AJ (2000) Cytokine activation of the HPA axis. Ann N Y Acad Sci 917(1):608–617
Spratt EG, Nicholas JS, Brady KT, Carpenter LA, Hatcher CR, Meekins KA et al (2012) Enhanced cortisol response to stress in children in autism. J Autism Dev Disorders 42(1):75–81
Taylor JL, Corbett BAJP (2014) A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 49:207–228
Seckl JRJE (2004) Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 151(Suppl 3):U49–U62
Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG (2006) Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiology 572(1):31
Gidaya NB, Lee BK, Burstyn I, Michael Y, Newschaffer CJ, Mortensen EL (2016) In utero Exposure to beta-2-adrenergic receptor agonist drugs and risk for autism spectrum disorders. Pediatrics 137(2):e20151316
Chua RXY, Tay MJY, Ooi DSQ, Siah KTH, Tham EH, Shek LP, Meaney MJ, Broekman BFP, Loo EXL (2021) Understanding the link between allergy and neurodevelopmental disorders: a current review of factors and mechanisms. Front Neurol 11:603571. https://doi.org/10.3389/fneur.2020.603571
Lord C, Petkova E, Hus V, Gan W, Lu F, Martin DM et al (2012) A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch Gener Psychiatry 69(3):306–313
Falkmer T, Anderson K, Falkmer M, Horlin C (2013) Diagnostic procedures in autism spectrum disorders: a systematic literature review. Eur Child Adolesc Psychiatry 22(6):329–340
Swan SH, Shaw GM, Schulman J (1992) Reporting and selection bias in case–control studies of congenital malformations. Epidemiology 3(4):356–363. https://doi.org/10.1097/00001648-199207000-00011
Author information
Authors and Affiliations
Contributions
All authors wrote the manuscript together and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seker, A., Qirko-Gurakuqi, A., Tabaku, M. et al. Maternal atopic conditions and autism spectrum disorder: a systematic review. Eur Child Adolesc Psychiatry (2023). https://doi.org/10.1007/s00787-023-02285-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00787-023-02285-7