Abstract
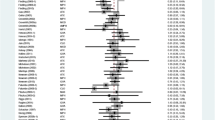
For patients with attention deficit hyperactivity disorder and comorbid conduct-dissocial disorder, a combination therapy of the psychostimulant methylphenidate and the antipsychotic risperidone may be prescribed. Case reports describe the occurrence of movement disorders under this combination therapy, but clinical trials had limited power to detect these events. This study aimed (1) to summarise published case reports and (2) to analyse pharmacovigilance data consisting of adverse drug event reports to elucidate these reactions. PubMed, Embase, and APA PsycInfo were used to retrieve case reports. For the pharmacovigilance data, aggregated information on individual case safety reports (ICSRs) within the database of suspected adverse drug events by the WHO were analysed. ICSRs were assessed for disproportionality in reporting. Thirteen published case reports (62% male) on movement disorders were identified, with ages between 5 and 15 years. Seven reports (54%) described incidents when risperidone was tapered down or switched to methylphenidate. From the WHO, we identified 25,556 ICSRs (16,118 for methylphenidate, 8,614 for risperidone, and 824 for both). Of these, 953 (5.9%), 1356 (15.7%), and 159 (19.3%) ICSRs reported movement disorders in association with methylphenidate, risperidone or both, respectively. The analyses on disproportionality showed an increased number of ICSRs with movement disorders when the two drugs were coded in combination. The potential of movement disorders as adverse effects might be amplified when methylphenidate and risperidone are used in combination. The results from the literature underline the necessity of caution and patient monitoring when risperidone dosing is modified during methylphenidate therapy.


Similar content being viewed by others
References
World Health Organization (2018) International Classification of Diseases, 11th Revision (ICD-11)
American Academy of Pediatrics, Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management (2011) ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022. https://doi.org/10.1542/peds.2011-2654
Matone M, Localio R, Huang Y-S, dosReis S, Feudtner C, Rubin D (2012) The relationship between mental health diagnosis and treatment with second-generation antipsychotics over time: a national study of US Medicaid-Enrolled Children. Health Serv Res 47:1836–1860. https://doi.org/10.1111/j.1475-6773.2012.01461.x
Kutcher S, Aman M, Brooks SJ, Buitelaar J, van Daalen E, Fegert J, Findling RL, Fisman S, Greenhill LL, Huss M, Kusumakar V, Pine D, Taylor E, Tyano S (2004) International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol 14:11–28. https://doi.org/10.1016/S0924-977X(03)00045-2
Yanofski J (2010) The Dopamine Dilemma. Psychiatry Edgmont 7:18–23
Sikirica V, Fridman M, Bruno A, Hodgkins P, Erder MH (2013) Concomitant pharmacotherapy of psychotropic medications in EU children and adolescents with attention-deficit/hyperactivity disorder. Drugs RD 13:271–280. https://doi.org/10.1007/s40268-013-0034-4
Betts KA, Sikirica V, Hodgkins P, Zhou Z, Xie J, DeLeon A, Erder MH, Wu EQ (2014) Period prevalence of concomitant psychotropic medication usage among children and adolescents with attention-deficit/hyperactivity disorder during 2009. J Child Adolesc Psychopharmacol 24:260–268. https://doi.org/10.1089/cap.2013.0107
Scholle O, Banaschewski T, Enders D, Garbe E, Riedel O (2018) Use and characteristics of antipsychotic/methylphenidate combination therapy in children and adolescents with a diagnosis of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 28:415–422. https://doi.org/10.1089/cap.2018.0024
Linton D, Barr AM, Honer WG, Procyshyn RM (2013) Antipsychotic and psychostimulant drug combination therapy in attention deficit/hyperactivity and disruptive behavior disorders: a systematic review of efficacy and tolerability. Curr Psychiatry Rep 15:355. https://doi.org/10.1007/s11920-013-0355-6
Aman MG, Binder C, Turgay A (2004) Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavior disorders, and subaverage IQ. J Child Adolesc Psychopharmacol 14:243–254. https://doi.org/10.1089/1044546041649020
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (2019) MedDRA®: medical dictionary for regulatory activities terminology, 22nd edn. https://www.meddra.org/
Uppsala Monitoring Centre (2019) UMC | Know more about VigiBase. In: UMC Know More VigiBase. https://www.who-umc.org/vigibase/vigibase/know-more-about-vigibase/. Accessed 23 Apr 2019
van Puijenbroek EP, Bate A, Leufkens HGM, Lindquist M, Orre R, Egberts ACG (2002) A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf 11:3–10. https://doi.org/10.1002/pds.668
Rothman KJ, Lanes S, Sacks ST (2004) The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf 13:519–523. https://doi.org/10.1002/pds.1001
RStudio Team (2019) RStudio: integrated development for R. RStudio, Boston
Core Team R (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Levine JB, Deneys ML, Benjamin S (2007) Dystonia with combined antipsychotic and stimulant treatment. J Am Acad Child Adolesc Psychiatry 46:665–666. https://doi.org/10.1097/chi.0b013e3180471b9d
Benjamin E, Salek S (2005) Stimulant-atypical antipsychotic interaction and acute dystonia. J Am Acad Child Adolesc Psychiatry 44:510–512. https://doi.org/10.1097/01.chi.0000159166.96967.13
Pérez CA, Garcia SS, Yu RD (2016) Extrapyramidal symptoms as a result of risperidone discontinuation during combination therapy with methylphenidate in a pediatric patient. J Child Adolesc Psychopharmacol 26:182–182. https://doi.org/10.1089/cap.2015.0225
Hollis CP, Thompson A (2007) Acute dyskinesia on starting methylphenidate after risperidone withdrawal. Pediatr Neurol 37:287–288. https://doi.org/10.1016/j.pediatrneurol.2007.05.017
Ince E, Algedik P, Demirdogen ES, Emul M, Demir T (2015) The relationship between acute dyskinesia with a single dose of methylphenidate and recent risperidone discontinuation in a child with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 25:378–379. https://doi.org/10.1089/cap.2014.0148
Sabuncuoglu O (2007) Risperidone-to-methylphenidate switch reaction in children: three cases. J Psychopharmacol (Oxf) 21:216–219. https://doi.org/10.1177/0269881107069466
Guler G, Yildirim V, Kutuk MO, Toros F (2015) Dystonia in an adolescent on risperidone following the discontinuation of methylphenidate: a case report. Clin Psychopharmacol Neurosci 13:115–117. https://doi.org/10.9758/cpn.2015.13.1.115
Teoh L, Allen H, Kowalenko N (2002) Drug-induced extrapyramidal reactions. J Paediatr Child Health 38:95–97. https://doi.org/10.1046/j.1440-1754.2002.00719.x
Feeney DJ, Klykylo W (1996) Risperidone and tardive dyskinesia. J Am Acad Child Adolesc Psychiatry 35:1421–1422. https://doi.org/10.1097/00004583-199611000-00006
Megens AAHP, Awouters FHL, Schotte A, Meert TF, Dugovic C, Niemegeers CJE, Leysen JE (1994) Survey on the pharmacodynamics of the new antipsychotic risperidone. Psychopharmacology 114:9–23. https://doi.org/10.1007/BF02245439
Jibson MD (2019) Second-generation antipsychotic medications: pharmacology, administration, and side effects. In: Marder S, Hermann R (eds) UpToDate. UpToDate, Waltham
Schmeichel BE, Berridge CW (2013) Neurocircuitry underlying the preferential sensitivity of prefrontal catecholamines to low-dose psychostimulants. Neuropsychopharmacology 38:1078–1084. https://doi.org/10.1038/npp.2013.6
Quinn N (1995) Fortnightly review: drug treatment of Parkinson’s disease. BMJ 310:575–579. https://doi.org/10.1136/bmj.310.6979.575
Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ, Kapur S, Zipursky RB, Wilson AA, Christensen BK, Seeman P (2000) Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology 152:174–180
Caster O, Juhlin K, Watson S, Norén GN (2014) Improved statistical signal detection in pharmacovigilance by combining multiple strength-of-evidence aspects in vigiRank. Drug Saf 37:617–628. https://doi.org/10.1007/s40264-014-0204-5
Beau-Lejdstrom R, Crook S, Spanu A, Yu T, Puhan MA (2019) Adverse drug reaction risk measures: a comparison of estimates from drug surveillance and randomised trials. Pharm Med 33:331–339. https://doi.org/10.1007/s40290-019-00287-y
Robottom BJ, Factor SA, Weiner WJ (2011) Movement disorders emergencies part 2: hyperkinetic disorders. Arch Neurol 68:719–724. https://doi.org/10.1001/archneurol.2011.117
Gilbert DL (2008) Drug-induced movement disorders in children. Ann N Y Acad Sci 1142:72–84. https://doi.org/10.1196/annals.1444.005
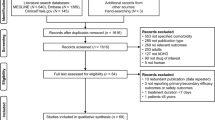
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Acknowledgements
MedDRA® trademark is owned by IFPMA on behalf of ICH. The information presented here does not represent the opinion of the UMC or the WHO.
Funding
The study was enabled by funds from the ETH Zurich, no external sources of funding were used to assist in the conduct of this study or the preparation of this article.
Author information
Authors and Affiliations
Contributions
Authors DS, SW, and AMB designed the study. DS managed the literature searches and analyses. DS and AMB undertook the statistical analysis, and DS wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest relevant to the content of this study. SW is a member of the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA). The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties.
Ethical standards
The data used only contained anonymised and aggregated clinical information.
Rights and permissions
About this article
Cite this article
Stämpfli, D., Weiler, S. & Burden, A. Movement disorders and use of risperidone and methylphenidate: a review of case reports and an analysis of the WHO database in pharmacovigilance. Eur Child Adolesc Psychiatry 30, 1047–1058 (2021). https://doi.org/10.1007/s00787-020-01589-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-020-01589-2