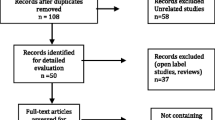
Abstract
Antipsychotics increase weight, BMI and waist size, particularly in pediatric patients. Switching antipsychotics is common practice, thus defining the risk for each antipsychotic in real-life settings can be important for clinical guidance. We conducted a meta-analysis on antipsychotic-related changes in body measures in pediatric observational studies. Of 934 publications found on PubMed, we analyzed 38, including nine treatment arms: no treatment, mixed antipsychotic treatment, first-generation antipsychotics, aripiprazole, clozapine, olanzapine, quetiapine, risperidone and ziprasidone. Changes in weight, BMI, BMI-Z and waist size were meta-analyzed according to the duration of clinical observations: 6, 12, > 12 months. Meta-regressions probed influencing factors. Weight in Kg was increased at 6, 12, > 12 months by olanzapine [+ 10.91, + 10.7, data not available (n/a)], mixed antipsychotic treatment (n/a, + 9.42, + 12.59), quetiapine (+ 5.84, n/a, n/a) and risperidone (+ 4.47, + 6.01, + 9.51) and without treatment (n/a, + 2.3, n/a). BMI was increased at 6, 12, > 12 months by olanzapine (+ 3.47, + 3.42, n/a), clozapine (n/a, + 3, n/a) mixed antipsychotic treatment (+ 3.37, + 2.95, + 3.32), risperidone (+ 2, + 2.13, + 2.16), quetiapine (+ 1.5, + 1.82, n/a), aripiprazole (n/a, + 1.7, + 2.1) and without treatment (n/a, + 0.75, n/a). BMI-Z was increased at 6, 12, > 12 months by olanzapine (+ 0.94, + 0.98, + 0.89), clozapine (n/a, + 0.8, n/a), risperidone (+ 0.62, + 0.61, + 0.48), quetiapine (+ 0.57, + 0.54, n/a), mixed antipsychotic treatment (+ 0.51, + 0.94, + 0.44), without treatment (n/a, + 0.37, n/a) and aripiprazole (no gain, + 0.31, n/a). Waist size in cm was increased at 6, 12 months by risperidone (+ 8.8, + 11.5), mixed antipsychotics treatment (+ 9.1, + 10.2) and quetiapine (+ 6.9, + 9.1). Overall, olanzapine and clozapine displayed maximum risk, followed by risperidone, quetiapine and aripiprazole (more risky at longer terms); ziprasidone was associated with no gains. No time-based trends emerged, suggesting a drug-specific risk magnitude. Meta-regressions evidenced variable roles for persistence in therapy and follow-up length, increased risk for drug-naïve patients, and a ceiling effect determined by higher baseline BMI/BMI-Z values.

Similar content being viewed by others
Data availability
Data are already public.
References
Olfson M, Druss BG, Marcus SC (2015) Trends in mental health care among children and adolescents. N Engl J Med 372:2029–2038
Eapen V, Shiers D, Curtis J (2013) Bridging the gap from evidence to policy and practice: reducing the progression to metabolic syndrome for children and adolescents on antipsychotic medication. Aust N Z J Psychiatry 47:435–442
Pisano S, Catone G, Veltri S et al (2016) Update on the safety of second generation antipsychotics in youths: a call for collaboration among paediatricians and child psychiatrists. Ital J Pediatr 42:51
Pozzi M, Pisano S, Bertella S et al (2016) Persistence in therapy with risperidone and aripiprazole in pediatric outpatients: a 2-year naturalistic comparison. J Clin Psychiatry 77:1601–1609
Rafaniello C, Pozzi M, Pisano S et al (2016) Second generation antipsychotics in 'real-life' paediatric patients. Adverse drug reactions and clinical outcomes of drug switch. Expert Opin Drug Saf 15:1–8
Kryzhanovskaya LA, Xu W, Millen BA et al (2012) Comparison of long-term (at least 24 weeks) weight gain and metabolic changes between adolescents and adults treated with olanzapine. J Child Adolesc Psychopharmacol 22:157–165
Baker RA, Pikalov A, Tran QV et al (2009) Atypical antipsychotic drugs and diabetes mellitus in the US Food and Drug Administration Adverse Event database: a systematic Bayesian signal detection analysis. Psychopharmacol Bull 42:11–31
Ajala O, Mold F, Boughton C et al (2017) Childhood predictors of cardiovascular disease in adulthood. A systematic review and meta-analysis. Obes Rev 18:1061–1070
Pringsheim T, Lam D, Ching H, Patten S (2011) Metabolic and neurological complications of second-generation antipsychotic use in children: a systematic review and meta-analysis of randomized controlled trials. Drug Saf 34:651–668
Almandil NB, Liu Y, Murray ML et al (2013) Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs 15:139–150
De Hert M, Dobbelaere M, Sheridan EM et al (2011) Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry 26:144–158
Tarricone I, Serretti A, Gozzi BF et al (2008) Metabolic side effects of second generation antipsychotic agents in antipsychotic-naïve patients: one-month prospective evaluation. Psychiatry Res 157:269–271
Tek C, Kucukgoncu S, Guloksuz S et al (2016) Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiatry 10:193–202
Leucht S, Cipriani A, Spineli L et al (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962
Pillinger T, McCutcheon RA, Vano L et al (2020) Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry 7:64–77
Krause M, Zhu Y, Huhn M et al (2018) Efficacy, acceptability, and tolerability of antipsychotics in children and adolescents with schizophrenia: a network meta-analysis. Eur Neuropsychopharmacol 28:659–674
Wakefield S, Aligeti M, Rachamallu V et al (2019) Metabolic monitoring of child and adolescent patients on atypical antipsychotics by psychiatrists and primary care providers. Am J Ther. https://doi.org/10.1097/MJT.0000000000000853
Sugawara N, Sagae T, Yasui-Furukori N et al (2018) Effects of nutritional education on weight change and metabolic abnormalities among patients with schizophrenia in Japan: a randomized controlled trial. J Psychiatr Res 97:77–83
Bozymski KM, Whitten JA, Blair ME et al (2018) Monitoring and treating metabolic abnormalities in patients with early psychosis initiated on antipsychotic medications. Community Ment Health J 54:717–724
Bak M, Fransen A, Janssen J et al (2014) Almost all antipsychotics result in weight gain: a meta-analysis. PLoS ONE 9:e94112
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013, https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Ratzoni G, Gothelf D, Brand-Gothelf A et al (2002) Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. J Am Acad Child Adolesc Psychiatry 41:337–343
Correll CU, Manu P, Olshanskiy V et al (2009) Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773
Menard ML, Thümmler S, Auby P, Askenazy F (2014) Preliminary and ongoing French multicenter prospective naturalistic study of adverse events of antipsychotic treatment in naive children and adolescents. Child Adolesc Psychiatry Ment Health 8:18
Moreno C, Merchán-Naranjo J, Alvarez M et al (2010) Metabolic effects of second-generation antipsychotics in bipolar youth: comparison with other psychotic and nonpsychotic diagnoses. Bipolar Disord 12:172–184
Kelly DL, Conley RR, Love RC et al (1998) Weight gain in adolescents treated with risperidone and conventional antipsychotics over six months. J Child Adolesc Psychopharmacol 8:151–159
Martin A, Landau J, Leebens P et al (2000) Risperidone-associated weight gain in children and adolescents: a retrospective chart review. J Child Adolesc Psychopharmacol 10:259–268
Zuddas A, Di Martino A, Muglia P, Cianchetti C (2000) Long-term risperidone for pervasive developmental disorder: efficacy, tolerability, and discontinuation. J Child Adolesc Psychopharmacol 10:79–90
Masi G, Cosenza A, Mucci M, Brovedani P (2003) A 3-year naturalistic study of 53 preschool children with pervasive developmental disorders treated with risperidone. J Clin Psychiatry 64:1039–1047
Kant R, Chalansani R, Chengappa KN, Dieringer MF (2004) The off-label use of clozapine in adolescents with bipolar disorder, intermittent explosive disorder, or posttraumatic stress disorder. J Child Adolesc Psychopharmacol 14:57–63
Castro-Fornieles J, Parellada M, Soutullo CA et al (2008) Antipsychotic treatment in child and adolescent first-episode psychosis: a longitudinal naturalistic approach. J Child Adolesc Psychopharmacol 18:327–336
Fraguas D, Merchán-Naranjo J, Laita P et al (2008) Metabolic and hormonal side effects in children and adolescents treated with second-generation antipsychotics. J Clin Psychiatry 69:1166–1175
Roy G, Bedard A, Desmarais PA et al (2010) Age-dependent metabolic effects of second-generation antipsychotics in second-generation antipsychotic-naïve French Canadian patients. J Child Adolesc Psychopharmacol 20:479–487
Cuerda C, Merchan-Naranjo J, Velasco C et al (2011) Influence of resting energy expenditure on weight gain in adolescents taking second-generation antipsychotics. Clin Nutr 30:616–623
Demb H, Valicenti-McDermott M, Navarro A, Ayoob KT (2011) The effect of long-term use of risperidone on body weight of children with an autism spectrum disorder. J Clin Psychopharmacol 31:669–670
Margari L, Matera E, Craig F et al (2013) Tolerability and safety profile of risperidone in a sample of children and adolescents. Int Clin Psychopharmacol 28:177–183
Arango C, Giráldez M, Merchán-Naranjo J et al (2014) Second-generation antipsychotic use in children and adolescents: a six-month prospective cohort study in drug-naïve patients. J Am Acad Child Adolesc Psychiatry 53(1179–90):1190.e1–4
Baeza I, Vigo L, de la Serna E et al (2017) The effects of antipsychotics on weight gain, weight-related hormones and homocysteine in children and adolescents: a 1-year follow-up study. Eur Child Adolesc Psychiatry 26:35–46
Ilies D, Huet AS, Lacourse E et al (2017) Long-term metabolic effects in french-canadian children and adolescents treated with second-generation antipsychotics in monotherapy or polytherapy: a 24-month descriptive retrospective study. Can J Psychiatry 62:827–836
Baeza I, de la Serna E, Calvo-Escalona R et al (2018) One-year prospective study of liver function tests in children and adolescents on second-generation antipsychotics: is there a link with metabolic syndrome? J Child Adolesc Psychopharmacol 28:463–473
Pozzi M, Pisano S, Marano G et al (2019) Weight-change trajectories of pediatric outpatients treated with risperidone or aripiprazole in a naturalistic setting. J Child Adolesc Psychopharmacol 29:133–140
Fleischhaker C, Heiser P, Hennighausen K et al (2008) Weight gain in children and adolescents during 45 weeks treatment with clozapine, olanzapine and risperidone. J Neural Transm (Vienna) 115:1599–1608
Degrauw RS, Li JZ, Gilbert DL (2009) Body mass index changes and chronic neuroleptic drug treatment for Tourette syndrome. Pediatr Neurol 41:183–186
Ghate SR, Porucznik CA, Said Q et al (2013) Association between second-generation antipsychotics and changes in body mass index in adolescents. J Adolesc Health 52:336–343
Szigethy E, Wiznitzer M, Branicky LA et al (1999) Risperidone-induced hepatotoxicity in children and adolescents? A chart review study. J Child Adolesc Psychopharmacol 9:93–98
Noguera A, Ballesta P, Baeza I et al (2013) Twenty-four months of antipsychotic treatment in children and adolescents with first psychotic episode: discontinuation and tolerability. J Clin Psychopharmacol 33:463–471
Nussbaum LA, Dumitraşcu V, Tudor A et al (2014) Molecular study of weight gain related to atypical antipsychotics: clinical implications of the CYP2D6 genotype. Rom J Morphol Embryol 55:877–884
O'Donoghue B, Schäfer MR, Becker J et al (2014) Metabolic changes in first-episode early-onset schizophrenia with second-generation antipsychotics. Early Interv Psychiatry 8:276–280
Matera E, Margari L, Palmieri VO et al (2017) Risperidone and cardiometabolic risk in children and adolescents: clinical and instrumental issues. J Clin Psychopharmacol 37:302–309
Hellings JA, Boehm D, Yeh HW et al (2011) Long-term aripiprazole in youth with developmental disabilities including autism. J Ment Health Res Intellect Disabil 4:40–52
Dominick K, Wink LK, McDougle CJ, Erickson CA (2015) A retrospective naturalistic study of ziprasidone for irritability in youth with autism spectrum disorder. J Child Adolesc Psychopharmacol 25:397–401
Wink LK, Early M, Schaefer T et al (2014) Body mass index change in autism spectrum disorders: comparison of treatment with risperidone and aripiprazole. J Child Adolesc Psychopharmacol 24:78–82
Ronsley R, Nguyen D, Davidson J, Panagiotopoulos C (2015) Increased risk of obesity and metabolic dysregulation following 12 months of second-generation antipsychotic treatment in children: a prospective cohort study. Can J Psychiatry 60:441–450
Sjo CP, Stenstrøm AD, Bojesen AB et al (2017) Development of metabolic syndrome in drug-naive adolescents after 12 months of second-generation antipsychotic treatment. J Child Adolesc Psychopharmacol 27:884–891
Schoemakers RJ, van Kesteren C, van Rosmalen J et al (2019) No differences in weight gain between risperidone and aripiprazole in children and adolescents after 12 months. J Child Adolesc Psychopharmacol 29:192–196
Calarge CA, Acion L, Kuperman S et al (2009) Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol 19:101–109
Del Castillo N, Zimmerman MB, Tyler B et al (2013) 759C/T variants of the serotonin (5-HT2C) receptor gene and weight gain in children and adolescents in long-term risperidone treatment. Clin Pharmacol Biopharm 2:110
Yoon Y, Wink LK, Pedapati EV et al (2016) Weight gain effects of second-generation antipsychotic treatment in autism spectrum disorder. J Child Adolesc Psychopharmacol 26:822–827
Pagsberg AK, Jeppesen P, Klauber DG et al (2017) Quetiapine extended release versus aripiprazole in children and adolescents with first-episode psychosis: the multicentre, double-blind, randomised tolerability and efficacy of antipsychotics (TEA) trial. Lancet Psychiatry 4:605–618
Brannsether B, Roelants M, Bjerknes R, Júlíusson PB (2011) Waist circumference and waist-to-height ratio in Norwegian children 4–18 years of age: reference values and cut-off levels. Acta Paediatr 100:1576–1582
Trikalinos TA, Olkin I (2012) Meta-analysis of effect sizes reported at multiple time points: a multivariate approach. Clinical Trials 9:610–620
Funding
This work was supported by the Regional Center of Pharmacovigilance of Lombardy (to EC), the Italian Medicines Agency, Agenzia Italiana del Farmaco (AIFA, to EC) and by the Italian Ministry of Health (Ricerca Corrente 2019–2020, to MP and MN; and Progetto Finalizzata RF-2016-02363761 to EC and AC) which are gratefully acknowledged. The funding public institutions had no role in any part of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest concerning the topic of this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pozzi, M., Ferrentino, R.I., Scrinzi, G. et al. Weight and body mass index increase in children and adolescents exposed to antipsychotic drugs in non-interventional settings: a meta-analysis and meta-regression. Eur Child Adolesc Psychiatry 31, 21–37 (2022). https://doi.org/10.1007/s00787-020-01582-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-020-01582-9