Abstract
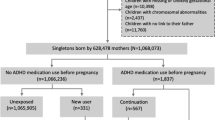
We aimed to examine the association between in utero exposure to β2AA and risk of attention-deficit/hyperactivity disorder (ADHD). We established a cohort of 672,265 children born from 1998 to 2008 in Denmark. Children were categorized as exposed if their mothers had redeemed a prescription of β2AA in pregnancy (from 30 days prior to conception until delivery). We identified children diagnosed with ADHD in the Danish National Hospital Register for the first time after his/her third birthday. Log-linear Poisson regression was used to estimate adjusted incidence rate ratio (aIRR) of ADHD. In total, 25,434 children were born to mothers who had redeemed a β2AA prescription in pregnancy. The exposed children had a 1.31-fold increased risk [aIRR = 1.30, 95% confidence interval (CI):1.20–1.42] of ADHD compared to unexposed children after adjusting for potential confounders. However, when extending the exposure window to 2 years prior to conception until delivery, exposure to maternal use of β2AA only before pregnancy, only during pregnancy, and both before and during pregnancy was associated with elevated risks of ADHD in children, with aIRRs of 1.31 (95% CI 1.22–1.40), 1.38 (95% CI 1.22–1.57), and 1.30 (95% CI 1.16–1.45), respectively. In mothers with a history of asthma, no association was observed between maternal use of β2AA during pregnancy and ADHD in offspring (aIRR = 0.92, 95% CI 0.74–1.15). In utero exposure to β2AA was associated with an increased risk of ADHD in children. However, it is more likely that confounding by indication, the underlying disorders or associated pathological conditions, may explain the association.
Similar content being viewed by others
References
Association AP (2013) Diagnostic and statistical manual of mental disorders, Fifth edition edn. American Psychiatric Association, Washington, DC
Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA (2014) ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 43(2):434–442. doi:10.1093/ije/dyt261
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164(6):942–948. doi:10.1176/ajp.2007.164.6.942
Biederman J, Petty CR, Monuteaux MC, Fried R, Byrne D, Mirto T, Spencer T, Wilens TE, Faraone SV (2010) Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case–control study. Am J Psychiatry 167(4):409–417. doi:10.1176/appi.ajp.2009.09050736
Dalsgaard S, Ostergaard SD, Leckman JF, Mortensen PB, Pedersen MG (2015) Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet (London, England) 385(9983):2190–2196. doi:10.1016/s0140-6736(14)61684-6
Lee SS, Humphreys KL, Flory K, Liu R, Glass K (2011) Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev 31(3):328–341. doi:10.1016/j.cpr.2011.01.006
Thapar A, Cooper M (2016) Attention deficit hyperactivity disorder. Lancet (London, England) 387(100):1240–1250. doi:10.1016/s0140-6736(15)00238-x
Thapar A, Cooper M, Eyre O, Langley K (2013) What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 54(1):3–16. doi:10.1111/j.1469-7610.2012.02611.x
Liew Z, Ritz B, Rebordosa C, Lee PC, Olsen J (2014) Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA pediatrics 168(4):313–320. doi:10.1001/jamapediatrics.2013.4914
Engeland A, Bramness JG, Daltveit AK, Ronning M, Skurtveit S, Furu K (2008) Prescription drug use among fathers and mothers before and during pregnancy. A population-based cohort study of 106,000 pregnancies in Norway 2004–2006. Br J Clin Pharmacol 65(5):653–660
NAEPP expert panel report (2005) Managing asthma during pregnancy recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol 115(1):34–46. doi:10.1016/j.jaci.2004.10.023
Schatz M, Dombrowski MP, Wise R, Momirova V, Landon M, Mabie W, Newman RB, Hauth JC, Lindheimer M, Caritis SN, Leveno KJ, Meis P, Miodovnik M, Wapner RJ, Paul RH, Varner MW, O’Sullivan MJ, Thurnau GR, Conway DL (2004) The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol 113(6):1040–1045. doi:10.1016/j.jaci.2004.03.017
Rocklin RE (2011) Asthma, asthma medications and their effects on maternal/fetal outcomes during pregnancy. Reprod Toxicol (Elmsford, NY) 32(2):189–197. doi:10.1016/j.reprotox.2011.05.023
Sodha RJ, Schneider H (1983) Transplacental transfer of beta-adrenergic drugs studied by an in vitro perfusion method of an isolated human placental lobule. Am J Obstet Gynecol 147(3):303–310
Bergman B, Bokstrom H, Borga O, Enk L, Hedner T, Wangberg B (1984) Transfer of terbutaline across the human placenta in late pregnancy. Eur J Respir Dis Suppl 134:81–86
Hsu CH, Robinson CP, Basmadjian GP (1994) Tissue distribution of 3H-terbutaline in rabbits. Life Sci 54(20):1465–1469
Rhodes MC, Seidler FJ, Abdel-Rahman A, Tate CA, Nyska A, Rincavage HL, Slotkin TA (2004) Terbutaline is a developmental neurotoxicant: effects on neuroproteins and morphology in cerebellum, hippocampus, and somatosensory cortex. J Pharmacol Exp Ther 308(2):529–537
Zerrate MC, Pletnikov M, Connors SL, Vargas DL, Seidler FJ, Zimmerman AW, Slotkin TA, Pardo CA (2007) Neuroinflammation and behavioral abnormalities after neonatal terbutaline treatment in rats: implications for autism. J Pharmacol Exp Ther 322(1):16–22
Slotkin TA, Seidler FJ (2007) Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Res Bull 73(4–6):301–309. doi:10.1016/j.brainresbull.2007.04.004
Connors SL, Crowell DE, Eberhart CG, Copeland J, Newschaffer CJ, Spence SJ, Zimmerman AW (2005) beta2-adrenergic receptor activation and genetic polymorphisms in autism: data from dizygotic twins. J Child Neurol 20(11):876–884
Croen LA, Connors SL, Matevia M, Qian Y, Newschaffer C, Zimmerman AW (2011) Prenatal exposure to beta2-adrenergic receptor agonists and risk of autism spectrum disorders. J Neurodev Disord 3(4):307–315
Gidaya NB, Lee BK, Burstyn I, Michael Y, Newschaffer CJ, Mortensen EL (2016) In utero exposure to beta-2-adrenergic receptor agonist drugs and risk for autism spectrum disorders. Pediatrics 137(2):1–8. doi:10.1542/peds.2015-1316
Pedersen CB, Gotzsche H, Moller JO, Mortensen PB (2006) The Danish civil registration system. A cohort of eight million persons. Dan Med Bull 53(4):441–449
Kildemoes HW, Sorensen HT, Hallas J (2011) The Danish national prescription registry. Scand J Public Health 39(7 Suppl):38–41. doi:10.1177/1403494810394717
Lynge E, Sandegaard JL, Rebolj M (2011) The Danish national patient register. Scand J Public Health 39(7 Suppl):30–33. doi:10.1177/1403494811401482
Mors O, Perto GP, Mortensen PB (2011) The Danish psychiatric central research register. Scand J Public Health 39(7 Suppl):54–57. doi:10.1177/1403494810395825
Knudsen LB, Olsen J (1998) The Danish medical birth registry. Dan Med Bull 45(3):320–323
Li J, Vestergaard M, Obel C, Cnattingus S, Gissler M, Olsen J (2011) Cohort profile: the Nordic perinatal bereavement cohort. Int J Epidemiol 40(5):1161–1167. doi:10.1093/ije/dyq127
Hadders-Algra M, Touwen BC, Huisjes HJ (1986) Long-term follow-up of children prenatally exposed to ritodrine. Br J Obstet Gynaecol 93(2):156–161
Pitzer M, Schmidt MH, Esser G, Laucht M (2001) Child development after maternal tocolysis with beta-sympathomimetic drugs. Child Psychiatry Hum Dev 31(3):165–182
Slotkin TA, Seidler FJ (2013) Terbutaline impairs the development of peripheral noradrenergic projections: potential implications for autism spectrum disorders and pharmacotherapy of preterm labor. Neurotoxicol Teratol 36:91–96. doi:10.1016/j.ntt.2012.07.003
Owens MY, Wallace KL, Mamoon N, Wyatt-Ashmead J, Bennett WA (2011) Absence of neurotoxicity with medicinal grade terbutaline in the rat model. Reprod Toxicol (Elmsford, NY) 31(4):447–453. doi:10.1016/j.reprotox.2011.01.001
Instanes JT, Halmoy A, Engeland A, Haavik J, Furu K, Klungsoyr K (2015) Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiatry. doi:10.1016/j.biopsych.2015.11.024
Hak E, de Vries TW, Hoekstra PJ, Jick SS (2013) Association of childhood attention-deficit/hyperactivity disorder with atopic diseases and skin infections? A matched case-control study using the General Practice Research Database. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol 111(2):102–106. doi:10.1016/j.anai.2013.05.023 (e102)
Mogensen N, Larsson H, Lundholm C, Almqvist C (2011) Association between childhood asthma and ADHD symptoms in adolescence–a prospective population-based twin study. Allergy 66(9):1224–1230. doi:10.1111/j.1398-9995.2011.02648.x
Romanos M, Gerlach M, Warnke A, Schmitt J (2010) Association of attention-deficit/hyperactivity disorder and atopic eczema modified by sleep disturbance in a large population-based sample. J Epidemiol Community Health 64(3):269–273. doi:10.1136/jech.2009.093534
Segman RH, Meltzer A, Gross-Tsur V, Kosov A, Frisch A, Inbar E, Darvasi A, Levy S, Goltser T, Weizman A, Galili-Weisstub E (2002) Preferential transmission of interleukin-1 receptor antagonist alleles in attention deficit hyperactivity disorder. Mol Psychiatry 7(1):72–74. doi:10.1038/sj/mp/4000919
Buske-Kirschbaum A, Schmitt J, Plessow F, Romanos M, Weidinger S, Roessner V (2013) Psychoendocrine and psychoneuroimmunological mechanisms in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder. Psychoneuroendocrinology 38(1):12–23. doi:10.1016/j.psyneuen.2012.09.017
Yarlagadda A, Alfson E, Clayton AH (2009) The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont (Pa: Township)) 6(11):18–22
Strickland AD (2014) Prevention of cerebral palsy, autism spectrum disorder, and attention deficit-hyperactivity disorder. Med Hypotheses 82(5):522–528. doi:10.1016/j.mehy.2014.02.003
Mohr-Jensen C, Vinkel Koch S, Briciet Lauritsen M, Steinhausen HC (2016) The validity and reliability of the diagnosis of hyperkinetic disorders in the Danish psychiatric central research registry. Eur Psychiatry J Assoc Eur Psychiatr 35:16–24. doi:10.1016/j.eurpsy.2016.01.2427
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jakob Christensen has received honoraria for serving on the Scientific Advisory Board of UCB Nordic and Eisai AB. Jakob Christensen has also received honoraria for giving lectures from UCB Nordic, and Eisai AB and has received funding for a trip from UCB Nordic. Other authors declare that they have no conflict of interest.
Funding
The study was supported by the National Natural Science Foundation of China (81428011), the Nordic Cancer Union (2013_129830, 2015_176673), the European Research Council (ERC-2010-StG-260242-PROGEURO), Danish Council for Independent Research (DFF-6110-00019A), and Karen Elise Jensen (2016). The funders have no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, H., Chen, J., Miao, M. et al. In utero exposure to β-2-adrenergic receptor agonist and attention-deficit/hyperactivity disorder in children. Eur Child Adolesc Psychiatry 26, 847–856 (2017). https://doi.org/10.1007/s00787-017-0956-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-017-0956-4