Abstract
Purpose
The aim of this study was to evaluate long-term clinical treatment with OROS® methylphenidate (MPH) (Concerta®) in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) who had been previously treated with immediate release (IR) MPH.
Methods
Subjects aged 6–16 years (n=105) who were stable on IR MPH (10–60 mg/day) were switched to 18, 36 or 54mg OROS® MPH once daily for 21 days, depending on prestudy MPH dose. Subjects who benefited from OROS® MPH could continue in a 12-month extension period. ADHD symptoms and treatment response were assessed by parents/caregivers and investigators.
Results
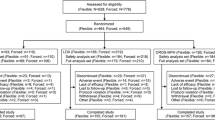
Out of 105 enrolled children, 101 completed the 21-day treatment phase. In all, 89 parents/caregivers (88.1%) wanted their child to continue with the study treatment into the extension phase, and 56 children (63 %) completed the 1-year trial. The parent/caregiver global assessment of satisfaction ranged from 49 to 69% during the extension phase, and 49 to 71% of investigators rated the treatment as adequate. Efficacy and satisfaction were found more commonly in patients in the older age group (10–16 years), those on a higher dose (36 mg or 54 mg) and with the predominantly inattentive ADHD subtype. OROS® MPH was well tolerated.
Conclusions
Children and adolescents can effectively and safely be switched from IR MPH to OROS® MPH with improved symptom control and compliance.
Similar content being viewed by others
References
American Academy of Child and Adolescent Psychiatry (1997) Practice parameter for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactive disorder. J Am Acad Child Adolesc Psychiatry 36 (Suppl): 85S–121S www.aacap.org/clinical/Adhdsum.htm.Accessed 10 May 2004
Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Benson RS, Bukstein O, Kinlan J, McClellan J, Rue D, Shaw JA, Stock S; American Academy of Child and Adolescent Psychiatry (2002) Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 41(2 Suppl):26S–49S
Kutcher S, Aman M, Brooks SJ, Buitelaar J, van Daalen E, Fegert J, Findling RL, Fisman S, Greenhill LL, Huss M, Kusumakar V, Pine D, Taylor E, Tyano S (2004) International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol 14:11–28
Loney J, Milich R (1982) Hyperactivity, inattention, and aggression in clinical practice. In: Wolraich M, Routh DK (eds) Advances in Development and Behavioral Pediatrics. JAI Press. Greenwich, CT, pp 113–147
Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M (1993) Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry 50:565–576
National Institute for Clinical Excellence (2002) Technology Appraisal Guidance-No 13. Guidance on the use of methylphenidate (Ritalin, Equasym) for attention-deficit/hyperactivity disorder (ADHD) in childhood. October. www.nice.org.uk/article.asp?a = 11667. Accessed 10 May 2004
Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, Chronis AM, Forehand GL, Nguyen CA, Hoffman MT, Lock TM, Fielbelkorn K, Coles EK, Panahon CJ, Steiner RL, Meichenbaum DL, Onyango AN,Morse GD (2001) Once- a-day Concerta methylphenidate versus threetimes-daily methylphenidate in laboratory and natural settings. Pediatrics 107:E105
Remschmidt H, Hoare P, Ettrich C, Rothenberger A, Santosh P, Schmit M, Spender Q, Tamhne R, Thompson M, Tinline C, Trott GE, Medori R (in press) Symptom control in children and adolescents with attention-deficit/hyperactivity disorder on switching from immediate-release MPH to OROS MPH: results of a 3-week open-label study. Eur Child Adolesc Psychiatry
Stein MA, Greenhill LL (2002) Oncedaily Concerta (MPH) for adolescents and adults with ADHD. Int J Neuropsychopharmacol 5(Suppl 1):S162
Wilens TE (2002) Treatment of ADHD with once-daily OROS methylphenidate: results from a long-term openlabel study. Presented at 155th Annual Meeting of the American Psychiatric Association, Philadelphia, PA, May 18–22
Wilens TE (personal communication) A multisite, controlled trial of OROS® methylphenidate (CONCERTA®) in the treatment of adolescents with attention-deficit/hyperactivity disorder
Wilens T, Pelham W, Stein MA,Conners CK, Abikoff H, Atkins M, August G, Greenhill L, McBurnett K, Palumbo D, Swanson J, Wolraich M (2003) ADHD treatment with once-daily OROS® methylphenidate: interim 12-month results from long-term open-label study. J Am Acad Child Adolesc Psychiatry 42:424–433
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoare, P., Remschmidt, H., Medori, R. et al. 12-month efficacy and safety of OROS® MPH in children and adolescents with attention-deficit/hyperactivity disorder switched from MPH. Europ.Child & Adolescent Psych 14, 305–309 (2005). https://doi.org/10.1007/s00787-005-0486-3
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00787-005-0486-3