Abstract
Objectives
Dental professionals are exposed to large amounts of dust particles during routine treatment and denture processing. This article provides a narrative review to investigate the most prevalent dust-related respiratory diseases among dental professionals and to discuss the effects of dental dust on human respiratory health.
Materials and methods
A literature search was performed in PubMed/Medline, Web of Science, and Embase for articles published between 1990 and 2022. Any articles on the occupational respiratory health effects of dental dust were included.
Results
The characterization and toxicity evaluation of dental dust show a correlation between dust exposure and respiratory system injury, and the possible pathogenic mechanism of dust is to cause lung injury and abnormal repair processes. The combination use of personal protective equipment and particle removal devices can effectively reduce the adverse health effects of dust exposure.
Conclusions
Dental dust should be considered an additional occupational hazard in dental practice. However, clinical data and scientific evidence on this topic are still scarce. Further research is required to quantify dust in the dental work environment and clarify its pathogenicity and potential toxicological pathways. Nonetheless, the prevention of dust exposure should become a consensus among dental practitioners.
Clinical relevance
This review provides dental practitioners with a comprehensive understanding and preventive advice on respiratory health problems associated with dust exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental professionals are constantly exposed to a variety of specific occupational hazards, including percutaneous exposure incidents (PEI), musculoskeletal disorders (MSD), contagious diseases, radiation, toxic effects associated with dental materials, respiratory diseases, and psychological problems [1]. The risk of bacterial and viral infections among dental personnel has been the focus of relevant research, especially during the coronavirus disease 2019 (COVID-19) outbreak [2, 3], and related guidelines have been developed to prevent occupational exposure [3]. However, dental dust, as a pervasive and potential health risk, has not attracted widespread attention.
Dust exposure is a well-known hazard to occupational health in industrial production. Pneumoconiosis, chronic bronchitis, emphysema, dust-related diffuse pulmonary fibrosis [4, 5], systemic connective tissue disease [6, 7], and even renal dysfunction [4, 5] have been linked to dust inhalation. In the daily dental work environment, we frequently observe visible dust particles floating around as dental materials and prostheses are being ground. Although numerous efforts have been made to improve the working environment, this phenomenon persists (Fig. 1). This inevitably raises concerns about the detrimental effects of dental dust particles on practitioners’ health.
The Centers for Disease Control and Prevention (CDC) previously issued a report on a group of dental professionals, including one dental technician and eight dentists, who were diagnosed with idiopathic pulmonary fibrosis (IPF) [8]. The etiology of IPF in these dental workers is not fully determined. In addition to viral infection and smoking, the authors suspected that occupational exposure to dust may be a causative factor. Some studies have shown the negative health effects of dental dust through in vitro characterization and toxicity tests [9, 10]. Generally, the toxicity of dental dust is correlated with the composition of dental materials and the physicochemical characteristics of the particles. First, dental dust retains the cytotoxicity of the dental material itself. For instance, the main components of porcelain dust were confirmed to be Si and O, whose cytotoxicity was similar to quartz and greater than that of Vitallium and polymethyl methacrylate (PMMA) resin dust [10]. Moreover, dimethacrylate monomers are known to be cytotoxic and may cause irritation, inflammation, and allergic reactions of the oral mucosa [11,12,13]. It has been shown that composite dust can continuously release residual monomers in the environment [14]. Second, the toxicity of dental dust can be caused directly by microparticles and nanoparticles. Their physicochemical characteristics, including particle diameter, shape, and surface area, are related to toxicity levels. Dust particles with small size and large surface area are more reactive and deposited deeper in the lungs, causing oxidative stress and inflammatory reactions [10].
The latest extensive review on the effects of dental dust evaluated the evidence from in vitro simulation and clinical studies [15] and indicated that composite dust should be considered an additional occupational hazard in dental practice. Nevertheless, the current research on dental dust is rare, and the possible health hazards, pathogenic mechanisms, and protective measures still lack detailed elaboration. The aim of this study is to perform a literature review to investigate the effects of dental dust on human respiratory health and to provide some references for dental practitioners.
Materials and methods
For this scoping review, a literature search was performed in PubMed/Medline, Web of Science, and Embase. The inclusion criteria encompassed articles published in the English language from 1990 up to 2022, focusing on the occupational health effects of dental dust on the respiratory system. The key search terms were divided into three parts: (i) Dust (MeSH), related: particles, particulate matter, dust particles; (ii) Dental Staff (MeSH), related: dentist, dental technician, dentistry, dental personnel, dental professional, dental assistant; and (iii) Respiratory Tract Diseases (MeSH), related: pneumoconiosis, respiratory tract neoplasms, respiratory symptoms, idiopathic pulmonary fibrosis, respiratory disease. The full electronic searches are shown in Table 1.
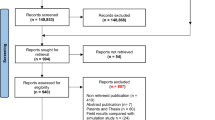
The search strategy identified 574 records. After removing duplicates, 38 articles were screened based on titles and abstracts. Also, a hand search was performed on the reference lists of all primary sources and eligible studies of this integrative review for additional relevant publications. Specifically, the studies involved case reports and case cohorts of respiratory disease in dental personnel and a limited number of in vitro studies of dental dust. However, the pathogenic mechanisms and prevention of dental dust are less well described. We believe that these two aspects are essential for a comprehensive understanding of the hazards of dental dust. Similar search procedures were then performed with the following search words “dust particles,” “pathogenic mechanism,” “health effect,” “personal protective equipment,” and “protective devices.” An additional 37 relevant articles have been included. In light of the small number of studies on this topic and the heterogeneity among studies, we chose a narrative review approach rather than a systematic review and meta-analysis.
Based on current surveys and research, we have focused on the following four issues:
-
(1) Common dust-related respiratory diseases among dental professionals.
-
(2) Dental dust characteristics in the workplace.
-
(3) Potential pathogenic mechanisms of dental dust on the respiratory system.
-
(4) Effective dust prevention and control methods in the dental practice setting.
Dust-related respiratory diseases of dental professionals
Dental technicians and dentists are exposed to composite dust and droplets every day of their careers. The risks associated with a dust-exposed environment, including a number of irritants, allergens, and potential carcinogens, should not be ignored. These airborne hazardous substances may contribute to the development of a variety of respiratory diseases.
Pneumoconiosis
Pneumoconiosis is the most commonly reported dust-related respiratory disease, and it was also the first occupational disease identified among dental professionals in 1939 [16]. Occupational pneumoconiosis is characterized by inflammation and fibrosis, and the most common symptoms are coughing, shortness of breath, and chest tightness [17]. It has been indicated that inhalation of dust containing heavy metals (particularly cobalt, chromium, and molybdenum), silica, gypsum, methyl methacrylate, and dental alginate may contribute to the development of pneumoconiosis in dental professionals [18, 19]. Several epidemiological studies reported a high incidence of pneumoconiosis among dental professionals, ranging from 4.5 to 47% after an average exposure time of 12.8 to 28.4 years (Table 2) [20,21,22,23,24,25,26,27,28].
A Turkey study found that the prevalence of pneumoconiosis was 10.1% among 893 dental technicians, and it was higher among men and those exposed to sandblasting [28]. Another study in Turkey also showed that a high proportion of pneumoconiosis patients were dental technologists. Among 60 patients diagnosed with pneumoconiosis between 2013 and 2015, 24 were dental technologists, comparable to ceramic workers (24 cases) and much higher than miners (3 cases) and marble cutters (3 cases) [29]. This indicates that dental technicians are at high risk of developing pneumoconiosis. Additionally, Dogan et al. performed a longitudinal study and found that the prevalence of pneumoconiosis increased from 13.8% (5/36) to 47.0% (9/19) among 36 dental technicians at the end of a 7-year follow-up [27]. However, 17 patients were lost during the follow-up period, and there was no control group in this cohort study. Thus, the large increase in prevalence in the study over 7 years needs to be interpreted with caution.
The disparities in the incidence of pneumoconiosis observed in the current studies may be attributable to the differences in occupational exposure duration and pneumoconiosis diagnostic criteria. In addition, the ventilation of the working environment and the level of personal protection awareness of dental professionals vary among different countries and dental offices. In general, pneumoconiosis is a significant occupational disease among dental professionals that requires attention.
Respiratory symptoms
Long-term exposure to dental dust is likely to trigger a variety of respiratory symptoms, including cough, nasal symptoms (runny nose or blocked or sneezing), pharyngitis, dyspnea, and hoarseness. Stoeva et al. conducted cross-sectional research among 4,675 dentists and found a 20.7% prevalence of work-related respiratory symptoms, which were associated with a lengthy period of employment, a history of atopic illness, and the female gender [30]. And a Finnish study reported that the prevalence of occupational-related respiratory symptoms among orthodontists (n = 141) and general dental practitioners (n = 208) was 28% and 18%, respectively [31]. The higher prevalence among orthodontists may be due to their frequent exposure to inhalable particles during orthodontic bracket debonding.
Additionally, it is reported that dental practitioners have a high rate of occupational respiratory allergies. They are constantly exposed to various airborne allergens and irritants in the workplace, such as disinfectants, methacrylates, and natural rubber latex (NRL) proteins [32]. Piirilä et al. and Lindström et al. reported occupational respiratory hypersensitivity caused by methacrylates among dental personnel [33, 34]. Common symptoms observed in these cases included occupational asthma, rhinitis, conjunctivitis, and laryngitis. Boudinar et al. assessed the occupation-related allergies of 584 French dentists through a self-administered questionnaire and found that 50.3% of the participants had allergies and 13.4% had occupational allergies [35]. Unfortunately, dental professionals are often unaware of the allergenic potential of dental materials and lack vigilance.
Respiratory cancer
The grinding of metal restorations produces a certain amount of heavy metal dust, in which beryllium and nickel compounds are classified as group 1 (carcinogenic), while metallic nickel, cobalt, and chromium [iii] are classified as group 2B (possibly carcinogenic) by the IARC [36]. In addition, exposure to asbestos fibers (commonly used as periodontal dressing binders) is assumed to be a risk factor for developing malignant mesothelioma. Reid et al. [37] reported the first case of asbestos-related malignant pleural mesothelioma in a dental clinic in 1991. Another recent study documented two more cases of mesothelioma in dental practitioners, which may be associated with occupational asbestos exposure [38].
Currently, the association between dental dust exposure and respiratory cancer remains controversial. A comprehensive review in 2021 [39] assessed the risk of asbestos exposure in dentistry and concluded that the use of asbestos-containing casting ring liners and/or periodontal dressing powder was not anticipated to increase asbestos-related disease risk. A Swedish study [40] discovered that the risk of lung cancer among dentists was comparable to that of the general population. This is consistent with the findings of another retrospective cohort study [41] that showed no statistically significant increase in the risk of lung cancer among Japanese male dentists. One possible explanation for this result is that dentists are exposed to relatively low levels of these carcinogens.
In order to define the role of dust exposure in the development of respiratory diseases, it is necessary to quantify the dust in the dental environment and explore its characteristics, pathogenicity, and potential pathogenic mechanisms.
Characteristics of dental dust
Dust is typically defined as airborne solid particles in a size range of 1 to 100 μm [42]. In the dental practice environment, dust is continuously generated during the processes of removing old restorations, grinding and polishing dental materials, and orthodontic bracket debonding [15]. The pathogenicity of dental dust in the respiratory system may be affected by its physicochemical characteristics, such as particle size, shape, concentration, and the presence or absence of nanoparticle release.
Size and shape
Particle size distribution and shape are critical parameters for evaluating the hazard of dental dust because they directly affect particle deposition in the respiratory tract. In occupational hygiene, aerodynamic diameter is typically used to describe particle size. And only dust particles with a smaller diameter can float in the air for an extended period, posing a significant risk to the respiratory system when continuously inhaled. Generally, particles with an aerodynamic diameter of 10 μm or less are inhalable and deposited in the upper respiratory tract. Those with aerodynamic diameters of less than 5 μm are considered respirable dust because they can reach the deep regions of the lungs [43].
A certain amount of evidence showed the generation of respirable dust during dental restorative procedures. Ilic et al. [44] used two different methodologies (laser diffraction and image analysis based on scanning electron microscopy) to characterize particles in dental laboratories. The measured particle size distribution indicated that almost all particles were respirable, with aerodynamic diameters ranging from 0.4–2 μm. Van Landuyt et al. [45] obtained comparable results in the laboratory by grinding composite blocks or rods to simulate routine prosthodontic practice and found that all composites produced respirable dust (< 5 μm) in vitro. Camassa et al. [9] characterized the collected grinding dust particles with scanning electron microscopy (SEM) and found that more than 80% of the particles had a minimum Feret diameter of less than 1 μm, which also meant that dental professionals were exposed to a substantial amount of respirable dust.
In addition to restorative dentistry, orthodontic treatment can generate inhalable dust. Brackets are bonded to the teeth using an orthodontic composite material and removed at the end of treatment. According to a number of studies [46,47,48,49,50], composite dust is typically produced during the enamel cleaning phase of orthodontic bracket debonding. These studies analyzed the effects of different speeds of the handpiece, cooling methods, curing procedures, and types of orthodontic brackets on particle production. Johnston et al. [49] found that using a high-speed handpiece with water cooling produced a higher particle concentration than using a slow-speed handpiece without water cooling. And these particles were all fully inhalable or respirable, with aerodynamic diameters of 4.24–10.5 μm. Gioka et al. [48] demonstrated that the size of particles produced by grinding chemically cured non-mixed orthodontic resin was much larger than that produced by light-cured adhesive resin. And Vig et al. [50] found no obvious difference in particle concentrations between conventional ceramic brackets and flash-free adhesive-coated brackets.
The shape of the particles can also affect their deposition in the respiratory tract. It was found that the total deposition amount of non-spherical particles, including micron-sized fibers and submicron-sized oblate disks, was appreciably higher than that of ideal spherical-shaped particles [51]. This is probably because spherical particles have less resistance in the air and settle faster, whereas non-spherical particles settle more slowly and have a longer suspension time. Wang et al. [10] observed the surface morphologies of three dental restorative materials before and after fine grinding (Fig. 2). The results suggested that the size and shape of PMMA grinding particles varied substantially. Particles from the porcelain group were cube-shaped with sharp edges, while those from the Vitallium group were sheet-like with irregular edges. Another study analyzed the elongation, roundness, and convexity of dental respirable dust [44], and indicated that smaller particles were more regular in shape than bigger particles. So far, there has been little discussion about the shape of dental composite dust, as shape remains one of the most difficult attributes to characterize and quantify.
Surface morphologies of dental dust. Scanning electron microscopy images of dental prosthesis grinding dust: PMMA (a), finely ground PMMA (b), Vitallium (c), finely ground Vitallium (d), porcelain (e), finely ground porcelain (f), and their composition: PMMA (g). Vitallium (h), porcelain (i). Reprinted with permission from ref. 10. Copyright (2020) Springer Nature
Release of nanoparticles
Dentistry is one of the most widely used fields of nanotechnology. And nanoscience research involves material scales ranging from 1 to 100 nm [52]. Due to their unique physical and chemical properties, nanoparticles are utilized in a wide range of dental materials, including dental composites, orthodontic adhesives, and root canal sealants. The incorporation of nanofillers into these materials enhances their physical, mechanical, and esthetic properties. For instance, dentures made of nanoceramic materials exhibit high hardness and strength, excellent corrosion resistance, and translucency [53]. Additionally, nanofillers can be added to polymethyl methacrylate (PMMA) in prosthodontics to significantly improve the transverse strength, surface hardness, and biological compatibility of the material and reduce its water sorption and solubility [54]. Furthermore, some nanofillers, such as TiO2 nanoparticles, can improve antibacterial properties without affecting the physical properties of the materials [55].
Despite the numerous benefits of nanocomposites, the release of nanoparticles during material grinding cannot be ignored, as nanoparticles are more hazardous to human health than larger particulate matter [56]. On the one hand, the small size of the nanoparticles enables them to be more efficiently deposited in the lungs, resulting in higher bio-persistence [57]. The surface area of inhaled nanoparticles, on the other hand, stimulates the generation of free radicals, which leads to oxidative stress and inflammatory reactions [58]. In addition, there is evidence that nanoparticles can translocate into the bloodstream or get into the brain through the olfactory epithelium [56, 59, 60]. Several studies have monitored the release of nanoparticles from dental nanocomposites, but the conclusions regarding the origin of the nanoparticles are inconsistent [9, 45, 61, 62]. Van Landuyt et al. first confirmed the release of nanoparticles during the polishing of dental composites and hypothesized that they originated from single nanofiller particles [45, 61]. Bradna et al. held different opinions on the source of nanoparticles [62]. They discovered that the size and content of filler particles had no effect on nanoparticle release but that diamond grain size and drilling speed did. The released nanoparticles might be the result of the thermal decomposition of the composite polymer matrix under the action of friction heat rather than of the filler nanoparticles. Camassa et al. [9] also believed that nanoparticles were produced by the thermal decomposition of the matrix and found that the concentration of ultra-fine particles produced by coarse and fine diamond drills operating at the same speed was identical, suggesting that the grinding speed may be an influencing factor. As the grinding speed increases, the process will generate more heat and thus release more nanoparticles. Based on the current viewpoint, it has been proposed that water cooling during grinding operations may trap nanoparticles within larger water droplets, thereby reducing the hazard by increasing particle size [45]. However, it should be taken into account that water cooling produces significant aerosols in the dental environment, which raises the risk of pathogen transmission.
Particle concentrations
High concentrations of dust particles released by dental clinics have a detrimental effect on indoor air quality, which may impair the respiratory function of patients and medical personnel and influence the occurrence and progression of dust-related diseases [63]. Researchers measured the daily PM10 (particulate matter < 10 μm) concentrations in several dental clinics and discovered that on the majority of days, the concentration values exceeded the Directive 1999/30/EC recommended limit of 50 μg/m3 [64]. And the increased concentration of particles is closely related to dental operations [65,66,67]. During working hours, the nanoparticle concentrations in dental laboratories and offices were significantly greater than during non-working hours [68]. Bernard et al. [65] discovered that a substantial number of sub-micrometer and super-micrometer PM particles were generated during dental treatment, and their average mass concentrations were 3.8 and 6.5 times higher than those during the unoccupied period, respectively. Sotiriou et al. [66] demonstrated that dental drilling treatments increased particle concentrations, with the majority of particles being less than 0.5 μm in size. And in the worst-case scenario of continuous high-speed drilling in a closed office without a protective device, it takes 95 min for the concentration of particles smaller than 0.5 μm to revert to background levels [67]. It can be speculated that dentists are exposed to excessive amounts of submicron particles and nanoparticles for extended periods.
The concentration of dust particles is not only a reflection of indoor air quality but also closely related to pathogenicity. Recent in vitro studies found that dental composite dust was toxic to cells and that the toxicity was concentration-dependent. Cokic et al. [69] evaluated the toxicity of whole composite dust fractions on human bronchial epithelial cells and found that non-specific biological effects such as decreased metabolic activity and pro-inflammatory IL-6 generation were observed only at sufficiently high concentrations of composite dust. In their follow-up study [70], the respirable fraction of the composite dust was collected and showed cytotoxic effects at the highest concentrations and mild genotoxicity at subtoxic concentrations (Fig. 3). Additionally, some researchers discovered that dental composite dust induced toxic effects on human bronchial epithelial cells HBEC-3KT in vitro after high doses and long-term exposures [9]. Wang et al. discovered that denture grinding dust was cytotoxic to RAW264.7 macrophages, and the release of reactive oxygen species and lactate dehydrogenase increased with time and concentration [10].
Cytotoxic effect of the respirable fraction of composite dust on human bronchial epithelial cells. Effects on the metabolic activity were analyzed by the WST-1 assay after 24 h (a) and 72 h (b) exposure, and effects on cell membrane integrity were determined by the LDH assay after 24 h (c) and 72 h (d). Reprinted with permission from ref. 70. Copyright (2020) Elsevier
It is essential to note that the simulated concentrations of dental dust in these studies do not accurately reflect actual exposure levels. The composition of the measured particles may be biased, especially in dental procedures combining water sprays and compressed air. Emitted water droplets, aerosolized saliva, and blood may be measured as solid particles in the air, which can affect the accuracy of the results. Therefore, dust concentration measurements in dental offices require further improvements, which are critical for determining the health risks associated with airborne dust, developing new dust prevention and control technologies, and evaluating the effectiveness of existing dust control measures.
Pathogenic mechanisms of dental dust
Inhalation is the primary route for the respiratory system to contact dust particles. To clarify the pathogenesis of dust-related respiratory diseases, it is important to understand the deposition and subsequent fate of dust particles, which are associated with the size and shape of the particles, as previously discussed in this study. Respirable dust, as well as nanoparticles, is deposited deeper in the respiratory system due to their small size. When dust particles settle in the alveolar area, they may cause chronic inflammation, epithelial damage, and further pulmonary fibrosis. According to the current literature, the pathogenic mechanisms of dust in the alveolar region can be reflected in two ways: causing lung injury and interfering with the normal repair process (Fig. 4).
Lung injury
Inhalation and deposition of dust particles cause oxidative stress and inflammatory reactions in affected areas, leading to lung injury [71]. Many researchers have established a link between oxidative stress and the pathogenesis of respiratory diseases, including COPD, asthma, and pulmonary fibrosis [72, 73]. When dust particles are deposited in the lung, reactive oxygen species (ROS), including superoxide anions, hydroxyl radicals, and hydrogen peroxide, among others, can be generated at the surface of the dust particles or by phagocytic cells [74]. These extremely reactive radicals react rapidly with biological macromolecules such as lipids, proteins, and DNA, causing structural and functional damage to the cell. When alveolar macrophages phagocytose dust particles, the induced oxidative stress will ultimately lead to the disintegration and death of the cells, and the particles released are then phagocytosed by other macrophages, forming a vicious cycle of continuous exposure to dust particles. Meanwhile, dust particles can activate specific molecular signals linked with oxidative stress, such as nuclear factor κB (NF-κB), nuclear factor erythroid 2 related factor 2 (Nrf2), mitogen-activated protein kinase (MAPK), and activator protein-1 (AP-1) [75]. During oxidative stress, NF-κB is activated in inflammatory cells and epithelial cells, resulting in the expression of numerous pro-inflammatory genes. A number of inflammatory and immunological genes are regulated by AP-1 in oxidant-mediated illnesses, and the MAPK family can also be susceptible to direct or indirect alterations by redox changes [76]. Nrf2 is an important transcription factor that regulates the cellular oxidative stress response. It has been demonstrated that PM10 induces oxidative damage due to the inhibition of the Nrf2-antioxidant signaling pathway [77].
Dust particles also stimulate macrophages and epithelial cells to release large amounts of pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor (TNF)-α, monocyte chemotactic protein (MCP)-1, and granulocyte–macrophage colony-stimulating factor (GM-CSF) [78]. These cytokines can induce the obvious recruitment of inflammatory cells to the alveolar wall and epithelium. Toxic mediators and hydrolases released by inflammatory cells compromise the integrity of the epithelium and contribute to tissue injury.
In addition to causing direct injury, dust particles may also lead to a reduction in mucociliary clearance. Yu et al. demonstrated that silica particles impaired mucociliary clearance by causing ultrastructural defects in airway cilia, overproduction of mucus, and alteration of MUC5B expression in the trachea [79]. As a result, toxic particles cannot be removed from the lungs by phagocytosis or mucociliary clearance, leading to continuous exposure to dust particles.
Abnormal injury/repair process
In response to lung injury induced by chronic inflammation and oxidative stress, epithelial cells initiate injury/repair processes that include mesenchymal cell recruitment and activation, myofibroblast secretion of extracellular matrix, re-epithelialization, and restoration of normal lung structure. All of these processes are tightly regulated by the interactions of various signaling pathways in the cells.
Continuous dust exposure activates cytokine/growth factor cascades, resulting in epithelial cell dysfunction and abnormal injury/repair processes [80]. Recent research showed that dust particle exposure induced macrophages and epithelial cells to secrete a large number of fibrogenic factors [81, 82]. Transforming growth factor (TGF-β1), a critical cytokine in the pathogenesis of pulmonary fibrosis, not only promotes epithelial-mesenchymal transformation but also signals to fibroblasts from the alveolar septum, converting them to myofibroblasts [83]. The myofibroblast is described as the classic pathological fibroblast phenotype in IPF lungs due to its ability to secrete an excessive amount of extracellular matrix [84]. Another cytokine produced by epithelial cells is platelet-derived growth factor (PDGF), which promotes fibroblast proliferation and ultimately results in lung fibrosis [85]. Simultaneously, dust particles can induce the release of interleukin-1 (IL-1) and tumor necrosis factor (TNF-α) by macrophages and epithelial cells, thereby promoting the expression of pro-fibrotic growth factors and their receptors [86]. TNF-α stimulates the production of TGF-β1, and IL-1β increases the expression of PDGF-AA and its receptor, PDGF receptor-α (PDGFR-α) [87, 88]. Activation of these pro-fibrosis factors results in abnormal injury/repair processes, such as alveolar epithelial-mesenchymal cell transformation, pathological fibroblast differentiation, and abnormal extracellular matrix deposition, which all contribute to the progression of pulmonary fibrosis.
Dust control
Dental dust particles should be considered an occupational hazard due to their potential pathogenicity after inhalation into the respiratory system. Dental professionals must strengthen the prevention consciousness of dust exposure and take effective protective measures to reduce the hazards of dental dust. This paper recommends the use of personal protective equipment and dust particle removal devices to protect dental professionals from the adverse effects of dust exposure.
Personal protective equipment (PPE)
Personal protective equipment, such as masks, face shields, gloves, and goggles, is one of the most basic precautions for dental professionals. These physical barriers can help limit direct exposure to dust particles.
Masks, the most common type of PPE, are classified into two categories: surgical masks and respiratory masks. The effectiveness of these masks is determined by their filtering capacity and structure [89]. A surgical mask is a disposable, loose-fitting mask that filters out approximately 80% of particles. An N95/FFP2 respirator is designed to fit tightly to the face and has a multi-layer polypropylene structure and electrostatic charges, providing a 95% filtering capacity for particles with a diameter of 0.3 μm [90]. Thus, respiratory masks offer superior protection to surgical masks in terms of material filtration and facial fit and are recommended for use when exposed to dust. Additionally, it is critical to ensure a proper fit and seal with the skin when wearing the mask and to avoid direct contact with the exterior of the mask when removing it.
Gloves are recognized as the second most common type of PPE [91]. They protect the dentists’ skin from potentially harmful substances and prevent cross-contamination during dental treatment, and the protective effect depends on the material of the gloves. Latex gloves have been routinely used in the dental profession, but they may cause allergic reactions, and a patch test is recommended prior to use. In contrast, nitrile and synthetic rubber gloves are safer and more durable and have been reported to provide the longest protection against methacrylate monomers [92]. In addition, goggles can be used to protect users from exposure to small-sized particles via the ocular pathways [93]. And the face shield is recommended to be used only as an accessory to other personal protective equipment [94]. Although the Centers for Disease Control and Prevention (CDC) recommends that dental workers wear face shields, there are also studies indicate that face shields are ineffective at preventing dental aerosol exposure [95].
Particle removal devices
A high-volume evacuator (HVE) is one type of suction device that draws a large volume of air over a period of time and reduces the exposure of patients and dental workers to airborne particles [96]. The use of HVE has been shown to eliminate the turbulent vortex in highly polluted areas near the mask and respiratory areas of dental professionals [97]. However, during restorative dentistry, HVE usually needs assistant cooperation and may obstruct the vision field of the operator.
Extraoral suction units (ESU) are high-airflow vacuum systems that can effectively reduce the concentration of particles between the patient’s mouth and the dentist’s eye level during dental treatment [98, 99]. Chavis et al. detected less spatter when the ESU was set to level 10 and placed 4 inches from the simulated patient’s mouth [98]. The advantage of ESU over HVE is that it does not require the cooperation of an assistant. In practical applications, the combination of HVE and ESU has proven to be more effective than HVE alone [95]. This is consistent with the finding of another study that HVE combined with ESU was superior to a saliva ejector alone or a combination of a saliva ejector and HVE for reducing aerosols and water droplets [100]. However, the use of these filtration devices inevitably presents some noise issues [101].
Ultraviolet (UV) treatment in the ventilation system and high-efficiency particulate air filters (HEPA) can effectively reduce the particles in the air [96]. HEPA can theoretically remove at least 99.97% of the airborne particles with a size of 0.3 μm, while the use of UV chambers in the ventilation system improves air quality and reduces irritants in the air. Nonetheless, these devices have some limitations. On the one hand, they are only effective if the particles are already present in the room’s air, and the removal procedure is time-consuming. On the other hand, the installation of these devices in the ventilation system requires engineering modifications, which are too expensive for most dental clinics.
Additionally, protective devices such as chairside acrylic adjustment cabinets and the X-Dent Box are available for specific needs such as extra-oral trimming and polishing dental prostheses [102, 103]. The chairside acrylic adjustment cabinet can collect acrylic fragments during prosthetic adjustment and shorten the time for aerosol to return to baseline levels [102], while the X-dent Box collects 65–90% of dust particles smaller than 5 μm [103].
It should be emphasized that no single piece of equipment or device can completely eliminate the risk of dust particles, and practitioners cannot rely solely on a single preventative measure. Consequently, it is strongly recommended that dental professionals wear PPE and cooperate with the use of ESUs and HVEs during procedures. In the case of extra-oral grinding and polishing dentures, the X-Dent Box and chairside acrylic adjustment cabinet can be used to collect additional harmful particles. If conditions permit, the use of HEPA and UV chambers in the ventilation system is encouraged. And it is necessary to strengthen the prevention awareness of practitioners. Dental professionals always ignore the adverse effects of dust because dust-related diseases tend to develop slowly [104]. They should be informed of the potential health hazards of dental dust in their daily work and the importance of prevention, and regular occupational health examinations should be conducted to facilitate early diagnosis and effective intervention.
Protective measures under the COVID-19 pandemic
As the COVID-19 pandemic has brought us to a new normal in dental practice, the need to comply with rigorous public health measures is more urgent than ever. Due to the nature of dental work and face-to-face interactions, dentists are considered to be at the highest risk of contracting COVID-19, much higher than nurses and general physicians [105]. During dental procedures, viruses can be transmitted via droplets or aerosols, and they can remain infectious on hands, objects, and surfaces for an extended period of time [106]. Therefore, protection for dental personnel is of utmost importance, and experts have come up with many practical guidelines for COVID-19 prevention [2, 3]. The recommended PPE consists of N95/FFP2 respirators, eye protection (goggles or procedure masks with a face shield), gowns, and gloves. In addition, the CDC recommends that engineering controls should be considered to properly maintain the ventilation system [3]. HEPA air filtration is recommended to improve ventilation and air cleaning, and ultraviolet germicidal irradiation (UVGI) in upper rooms is applied as a supplement to enhance air sterilization. These recommended PPE and engineering controls are also effective measures we have mentioned above for reducing dental dust exposure, indicating that these higher levels of protective measures have general applicability for reducing dental related occupational exposures. When dental practitioners take these protective measures against COVID-19, their exposure risk to dental dust is consequently reduced.
Given the shortage of resources in the world today, it has been suggested that the use of higher levels of protection should be contingent on the spread of COVID-19 in the community [107]. When transmission rates are low or pandemics are mitigated, these measures would be a waste of resources and place an additional burden on resource-limited countries. Nevertheless, we insist that dental staff should be fully outfitted with basic PPE, including high-standard N95/FFP2 respirators, eye protection, robes, and gloves. Dental professionals should remain vigilant at all times and adequately protect against the risks of occupational exposure in the dental setting.
Limitations
The health effect of dental dust has not caused extensive concern. Due to the limited number of current studies and the heterogeneity among them, we did not apply appraisal tools to evaluate the quality of the included evidence, and only performed a qualitative analysis of dust hazards in this narrative review. There is still some important work to be done on dental dust in the further.
The first is to quantify the actual dust exposure of dental personnel. Not all of the dust particles that enter the respiratory tract are deposited in the lungs, and some of them are exhaled. The amount of dental dust in the environment may not reflect the actual level of exposure and cumulation. In order to determine the true exposure dose, comprehensive studies are needed to investigate the relationship between dental dust characterization and respiratory deposition. In addition, future research should include routine real-time monitoring of dental dust, which will provide valuable information to clarify the association between dental dust and the development of respiratory tract diseases in dental professionals. Current dust monitoring equipment only analyzes the overall concentration of particulate matter and cannot describe the dust components and their respective proportions. Consequently, the development of new instruments and devices to quantify dust concentration and composition will be key to evaluating dental dust exposure.
The second is to conduct dust toxicology research at the level of tissues, organs, and living animals. Current studies on the toxicity of dental dust are restricted to in vitro cellular studies. To examine the effect of dental dust at the organ level, the lung-on-a-chip model can be used [108]. The device can predict the absorption of dental dust and gauge potential changes in lung function by measuring surfactant production and the permeability of the alveolar barrier. Also, it is necessary to establish a unified animal model for systematic qualitative and quantitative analysis, so as to provide a theoretical basis for further research on the pathogenic mechanisms of dental dust and clinical dust prevention.
Conclusion
Within the limitations of the present review, dust exposure is a potential risk factor for respiratory disease in dental professionals. Nevertheless, the scientific evidence from the eligible studies is not enough to draw a clear conclusion. More well-designed studies are needed in the future to carefully elucidate the hazards of dental dust and identify effective strategies for reducing dust exposure. Furthermore, dental personnel should keep alert for occupational dust hazards and strengthen awareness of prevention in daily work.
References
Mohammed NS, Shaik MA (2013) Occupational hazards in modern dentistry. Int J Exp Dental Sci 2:33. https://doi.org/10.5005/jp-journals-10029-1037
Meng L, Hua F, Bian Z (2020) Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res 99:481–487. https://doi.org/10.1177/0022034520914246
National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. (2020) Guidance for dental settings: interim infection prevention and control guidance for dental settings during the COVID-19 response. CDC’s Website. https://stacks.cdc.gov/view/cdc/88256 Accessed 21 December 2022
Leung CC, Yu ITS, Chen W (2012) Silicosis. Lancet 379:2008–2018. https://doi.org/10.1016/S0140-6736(12)60235-9
Committee A (1997) Adverse effects of crystalline silica exposure. American Thoracic Society Committee of the Scientific Assembly on Environmental and Occupational Health. Am J Resp Crit Care 155:761–768. https://doi.org/10.1164/ajrccm.155.2.9032226
Shtraichman O, Blanc PD, Ollech JE, Fridel L, Fuks L, Fireman E, Kramer MR (2015) Outbreak of autoimmune disease in silicosis linked to artificial stone. Occup Med-Oxford 65:444–450. https://doi.org/10.1093/occmed/kqv073
Turner MT, Samuel SR, Silverstone EJ, Yates DH (2020) Silica exposure and connective tissue disease: an underrecognized association in three Australian artificial stone workers. Am J Resp Crit Care 201:378–380. https://doi.org/10.1164/rccm.201905-1057LE
Nett RJ, Cummings KJ, Cannon B, Cox-Ganser J, Nathan SD (2018) Dental personnel treated for idiopathic pulmonary fibrosis at a tertiary care center - Virginia, 2000–2015. Mmwr-Morbid Mortal W 67:270–273. https://doi.org/10.15585/mmwr.mm6709a2
Camassa LMA, Ervik TK, Zegeye FD, Mdala I, Valen H, Ansteinsson V, Zienolddiny S (2021) Characterization and toxicity evaluation of air-borne particles released by grinding from two dental resin composites in vitro. Dent Mater 37:1121–1133. https://doi.org/10.1016/j.dental.2021.03.011
Wang W, Li T, Luo X, Zhan KG, Cao N, Liu K, Li X, Zhu Y (2020) Cytotoxic effects of dental prosthesis grinding dust on RAW264. 7 cells. Sci Rep-UK 10:1–10. https://doi.org/10.1038/s41598-020-71485-x
Carli E, Pasini M, Lardani L, Giuca G, Miceli M (2021) Impact of self-ligating orthodontic brackets on dental biofilm and periodontal pathogens in adolescents. J Biol Regul Homeost Agents 35.3 Suppl 1:107–115.https://doi.org/10.23812/21-3supp1-13
Pagano S, Lombardo G, Caponi S, Costanzi E, Di Michele A, Bruscoli S, Xhimitiku I, Coniglio M, Valenti C, Mattarelli M, Rossi G, Cianetti S, Marinucci L (2021) Bio-mechanical characterization of a CAD/CAM PMMA resin for digital removable prostheses. Dent Mater 37:e118–e130. https://doi.org/10.1016/j.dental.2020.11.003
Goiato MC, Freitas E, dos Santos D, de Medeiros R, Sonego M (2015) Acrylic resin cytotoxicity for denture base—literature review. Adv Clin Exp Med 24:679–686. https://doi.org/10.17219/acem/33009
Cokic SM, Duca RC, Godderis L, Hoet PH, Seo JW, Van Meerbeek B, Van Landuyt KL (2017) Release of monomers from composite dust. J Dent 60:56–62. https://doi.org/10.1016/j.jdent.2017.02.016
Iliadi A, Koletsi D, Eliades T, Eliades G (2020) Particulate production and composite dust during routine dental procedures. a systematic review with meta-analyses. Materials 13. https://doi.org/10.3390/ma13112513
Siltzbach LE (1939) The silicosis hazard in mechanical dentistry. JAMA-J Am Med Assoc 113:1116–1119. https://doi.org/10.1001/jama.1939.02800370032007
Qi XM, Luo Y, Song MY, Liu Y, Shu T, Pang JL, Wang J, Wang C (2021) Pneumoconiosis: current status and future prospects. Chin Med J-Peking 134:898–907. https://doi.org/10.1097/cm9.0000000000001461
Barbieri PG, Somigliana A, Carradori G (2020) Severe silicosis due to diatomaceous earth in dental alginate: a necropsy study. Med Lav 111:222–231. https://doi.org/10.23749/mdl.v111i3.9742
Cocârţă DM, Prodana M, Demetrescu I, Lungu PEM, Didilescu AC (2021) Indoor air pollution with fine particles and implications for workers’ health in dental offices: A Brief Review. Sustain-Basel 13:599. https://doi.org/10.3390/su13020599
Rom WN, Lockey JE, Lee JS, Kimball AC, Bang KM, Leaman H, Johns RE Jr, Perrota D, Gibbons HL (1984) Pneumoconiosis and exposures of dental laboratory technicians. Am J Public Health 74:1252–1257. https://doi.org/10.2105/AJPH.74.11.1252
Sherson D, Maltbaek N, Olsen O (1988) Small opacities among dental laboratory technicians in Copenhagen. Occup Environ Med 45:320–324. https://doi.org/10.1136/oem.45.5.320
Choudat D, Triem S, Weill B, Vicrey C, Ameille J, Brochard P, Letourneux M, Rossignol C (1993) Respiratory symptoms, lung function, and pneumoconiosis among self employed dental technicians. Occup Environ Med 50:443–449. https://doi.org/10.1136/oem.50.5.443
Selden A, Persson B, Bornberger-Dankvardt S, Winström L, Bodin L (1995) Exposure to cobalt chromium dust and lung disorders in dental technicians. Thorax 50:769–772. https://doi.org/10.1136/thx.50.7.769
Froudarakis ME, Voloudaki A, Bouros D, Drakonakis G, Hatzakis K, Siafakas NM (1999) Pneumoconiosis among Cretan dental technicians. Respiration 66:338–342. https://doi.org/10.1159/000029404
Radi S, Dalphin J, Manzoni P, Pernet D, Leboube M, Viel J (2002) Respiratory morbidity in a population of French dental technicians. Occup Environ Med 59:398–404. https://doi.org/10.1136/oem.59.6.398
Cimrin A, Kömüs N, Karaman C, Tertemiz KC (2009) Pneumoconiosis and work-related health complaints in Turkish dental laboratory workers. Tuberk Torak 57:282–288. https://pubmed.ncbi.nlm.nih.gov/19787467/
Dogan DÖ, Berk S, Gumus C, Ozdemir AK, Akkurt I (2013) A longitudinal study on lung disease in dental technicians: what has changed after seven years? Int J Occup Med Env 26:693–701. https://doi.org/10.2478/s13382-013-0140-0
Ergün D, Ergün R, Özdemir C, Öziş TN, Yilmaz H, Akkurt İ (2014) Pneumoconiosis and respiratory problems in dental laboratory technicians: analysis of 893 dental technicians. Int J Occup Med Env 27:785–796. https://doi.org/10.2478/s13382-014-0301-9
Alici NS, Cimrin A, Coskun Beyan A (2016) Pneumoconiosis in different sectors and their differences in Turkey. Tuberk Torak 64:275–282. https://doi.org/10.5578/tt.27995
Stoeva I (2021) Respiratory symptoms of exposure to substances in the workplace among Bulgarian dentists. Community Dent Oral 49:128–135. https://doi.org/10.1111/cdoe.12584
Kerosuo E, Kerosuo H, Kanerva L (2000) Self-reported health complaints among general dental practitioners, orthodontists, and office employees. Acta Odontol Scand 58:207–212. https://doi.org/10.1080/000163500750051755
Hamann CP, Rodgers PA, Sullivan KM (2004) Occupational allergens in dentistry. Curr Opin Allergy Cl 4:403–409. https://doi.org/10.1097/00130832-200410000-00012
Piirilä P, Kanerva L, Keskinen H, Estlander T, Hytönen M, Tuppurainen M, Nordman H (1998) Occupational respiratory hypersensitivity caused by preparations containing acrylates in dental personnel. Clin Exp Allergy: J Br Soc Allergy Clin Immunol 28:1404–1411. https://doi.org/10.1046/j.1365-2222.1998.00400.x
Lindstrom M, Alanko K, Keskinen H, Kanerva L (2002) Dentist’s occupational asthma, rhinoconjunctivitis, and allergic contact dermatitis from methacrylates. Allergy 57:543–545. https://doi.org/10.1034/j.1398-9995.2002.03199.x
Boudinar L, Offner D, Jung S (2021) Occupational allergies in dentistry: a cross-sectional study in a group of French dentists. Oral 1:39–152. https://doi.org/10.3390/oral1020014
Anttila A (2006) International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans. Lyon, France
Reid A, Causton B, Jones J (1991) Malignant mesothelioma after exposure to asbestos in dental practice. Lancet 338:696. https://doi.org/10.1016/0140-6736(91)91273-W
Mensi C, Ciullo F, Barbieri GP, Riboldi L, Somigliana A, Rasperini G, Pesatori AC, Consonni D (2017) Pleural malignant mesothelioma in dental laboratory technicians: A case series. Am J Ind Med 60:443–448. https://doi.org/10.1002/ajim.22716
Ierardi AM, Mathis C, Urban A, Jacobs N, Finley B, Gaffney S (2021) Potential airborne asbestos exposures in dentistry: a comprehensive review and risk assessment. Crit Rev Toxicol 51:301–327. https://doi.org/10.1080/10408444.2021.1910624
Eklund G, Izikowitz L, Molin C (1990) Malignant tumours in Swedish dental personnel: a comparative study with the total population as well as with some specific occupational groups. Swed Dent J 14:249–254. https://pubmed.ncbi.nlm.nih.gov/2096472/
Nishio N, Tanaka H, Tsukuma H, Tokunaga R (2004) Lung cancer risk in male dentists: a retrospective cohort study in Japan, 1964–1997. J Occup Health 46:37–42. https://doi.org/10.1539/joh.46.37
World Health Organization. Occupational and Environmental Health Team (1999) Hazard prevention and control in the work environment:airborne dust. World Health Organization. https://apps.who.int/iris/handle/10665/66147
Koehler KA, Volckens J (2013) Development of a sampler to estimate regional deposition of aerosol in the human respiratory tract. Ann Occup Hyg 57:1138–1147. https://doi.org/10.1093/annhyg/met041
Ilic M, Budak I, Vasic MV, Nagode A, Kozmidis-Luburic U, Hodolic J, Puskar T (2015) Size and shape particle analysis by applying image analysis and laser diffraction - Inhalable dust in a dental laboratory. Measurement 66:109–117. https://doi.org/10.1016/j.measurement.2015.01.028
Van Landuyt KL, Yoshihara K, Geebelen B, Peumans M, Godderis L, Hoet P, Van Meerbeek B (2012) Should we be concerned about composite (nano-)dust? Dent Mater 28:62–70. https://doi.org/10.1016/j.dental.2012.08.011
Ireland AJ, Moreno T, Price R (2003) Airborne particles produced during enamel cleanup after removal of orthodontic appliances. Am J Orthod Dentofac 124:683–686. https://doi.org/10.1016/S0889-5406(03)00623-1
Day CJ, Price R, Sandy JR, Ireland AJ (2008) Inhalation of aerosols produced during the removal of fixed orthodontic appliances: a comparison of 4 enamel cleanup methods. Am J Orthod Dentofac 133:11–17. https://doi.org/10.1016/j.ajodo.2006.01.049
Gioka C, Eliades T, Zinelis S, Pratsinis H, Athanasiou AE, Eliades G, Kletsas D (2009) Characterization and in vitro estrogenicity of orthodontic adhesive particulates produced by simulated debonding. Dent Mater 25:376–382. https://doi.org/10.1016/j.dental.2008.08.010
Johnston NJ, Price R, Day CJ, Sandy JR, Ireland AJ (2009) Quantitative and qualitative analysis of particulate production during simulated clinical orthodontic debonds. Dent Mater 25:1155–1162. https://doi.org/10.1016/j.dental.2009.04.002
Vig P, Atack NE, Sandy JR, Sherriff M, Ireland AJ (2019) Particulate production during debonding of fixed appliances: Laboratory investigation and randomized clinical trial to assess the effect of using flash-free ceramic brackets. Am J Orthod Dentofac 155:767–778. https://doi.org/10.1016/j.ajodo.2019.02.010
Sturm R (2012) A computer model for the simulation of nonspherical particle dynamics in the human respiratory tract. Phys Res Int 2012. https://doi.org/10.1155/2012/142756
Barot T, Rawtani D, Kulkarni P (2021) Nanotechnology-based materials as emerging trends for dental applications. Rev Adv Mater Sci 60:173–189. https://doi.org/10.1515/rams-2020-0052
Wang W, Liao S, Zhu Y, Liu M, Zhao Q, Fu Y (2015) Recent applications of nanomaterials in prosthodontics. J Nanomater 2015. https://doi.org/10.1155/2015/408643
Jasim BS, Ismail IJ (2014) The effect of silanized alumina nano-fillers addition on some physical and mechanical properties of heat cured polymethyl methacrylate denture base material. J Baghdad Coll Dent 26:18–23. https://doi.org/10.12816/0015190
Poosti M, Ramazanzadeh B, Zebarjad M, Javadzadeh P, Naderinasab M, Shakeri MT (2013) Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur J Orthodont 35:676–679. https://doi.org/10.1093/ejo/cjs073
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Persp 113:823–839. https://doi.org/10.1289/ehp.7339
Byrne JD, Baugh JA (2008) The significance of nanoparticles in particle-induced pulmonary fibrosis. McGill J Med 11:43–50. https://doi.org/10.26443/mjm.v11i1.455
Bonner JC (2007) Lung fibrotic responses to particle exposure. Toxicol Pathol 35:148–153. https://doi.org/10.1080/01926230601060009
Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, Schins R, Stone V, Kreyling W, Lademann J (2006) The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol 3:1–35. https://doi.org/10.1186/1743-8977-3-11
Stone V, Johnston H, Clift MJ (2007) Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions. IEEE T Nanobiosci 6:331–340. https://doi.org/10.1109/TNB.2007.909005
Van Landuyt KL, Hellack B, Van Meerbeek B, Peumans M, Hoet P, Wiemann M, Kuhlbusch T, Asbach C (2014) Nanoparticle release from dental composites. Acta Biomater 10:365–374. https://doi.org/10.1016/j.actbio.2013.09.044
Bradna P, Ondrackova L, Zdimal V, Navratil T, Pelclova D (2017) Detection of nanoparticles released at finishing of dental composite materials. Monatsh Chem 148:531–537. https://doi.org/10.1007/s00706-016-1912-6
Abakay A, Atilgan S, Abakay O,Atalay Y, Guven S, Yaman F, Tanrikulu A (2013) Frequency of respiratory function disorders among dental laboratory technicians working under conditions of high dust concentration. Eur Rev Med Pharmaco 17:809–814. https://pubmed.ncbi.nlm.nih.gov/23609365/
Helmis C, Tzoutzas J, Flocas H, Halios C, Stathopoulou O, Assimakopoulos V, Panis V, Apostolatou M, Sgouros G, Adam E (2007) Indoor air quality in a dentistry clinic. Sci Total Environ 377:349–365. https://doi.org/10.1016/j.scitotenv.2007.01.100
Polednik B (2021) Exposure of staff to aerosols and bioaerosols in a dental office. Build Environ 187:107388. https://doi.org/10.1016/j.buildenv.2020.107388
Sotiriou M, Ferguson SF, Davey M, Wolfson JM, Demokritou P, Lawrence J, Sax SN, Koutrakis P (2008) Measurement of particle concentrations in a dental office. Environ Monit Assess 137:351–361. https://doi.org/10.1007/s10661-007-9770-7
Razavi M, Butt ZA, Chen H, Tan Z (2021) In situ measurement of airborne particle concentration in a real dental office: Implications for disease transmission. Int J Env Res Pub He 18:8955. https://doi.org/10.3390/ijerph18178955
Lang A, Ovsenik M, Verdenik I, Remškar M, Oblak Č (2018) Nanoparticle concentrations and composition in a dental office and dental laboratory: a pilot study on the influence of working procedures. J Occup Environ Hyg 15:441–447. https://doi.org/10.1080/15459624.2018.1432864
Cokic SM, Hoet P, Godderis L, Wiemann M, Asbach C, Reichl FX, De Munck J, Van Meerbeek B, Van Landuyt KL (2016) Cytotoxic effects of composite dust on human bronchial epithelial cells. Dent Mater 32:1482–1491. https://doi.org/10.1016/j.dental.2016.09.010
Cokic SM, Ghosh M, Hoet P, Godderis L, Van Meerbeek B, Van Landuyt KL (2020) Cytotoxic and genotoxic potential of respirable fraction of composite dust on human bronchial cells. Dent Mater 36:270–283. https://doi.org/10.1016/j.dental.2019.11.009
Feng SL, Gao D, Liao F, Zhou FR, Wang XM (2016) The health effects of ambient PM2.5 and potential mechanisms. Ecotox Environ Safe 128:67–74. https://doi.org/10.1016/j.ecoenv.2016.01.030
Andreadis AA, Hazen SL, Comhair SAA, Erzurum SC (2003) Oxidative and nitrosative events in asthma. Free Radical Bio Med 35:213–225. https://doi.org/10.1016/S0891-5849(03)00278-8
Lin J-L, Thomas PS (2010) Current perspectives of oxidative stress and its measurement in chronic obstructive pulmonary disease. COPD 7:291–306. https://doi.org/10.3109/15412555.2010.496818
Fubini B, Hubbard A (2003) Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radical Bio Med 34:1507–1516. https://doi.org/10.1016/S0891-5849(03)00149-7
Bhargava A, Shukla A, Bunkar N, Shandilya R, Lodhi L, Kumari R, Gupta PK, Rahman A, Chaudhury K, Tiwari R (2019) Exposure to ultrafine particulate matter induces NF-κβ mediated epigenetic modifications. Environ Pollut 252:39–50. https://doi.org/10.1016/j.envpol.2019.05.065
Leikauf GD, Kim S-H, Jang A-S (2020) Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med 52:329–337. https://doi.org/10.1038/s12276-020-0394-0
Radan M, Dianat M, Badavi M, Mard SA, Bayati V, Goudarzi G (2019) In vivo and in vitro evidence for the involvement of Nrf2-antioxidant response element signaling pathway in the inflammation and oxidative stress induced by particulate matter (PM10): the effective role of gallic acid. Free Radical Res 53:210–225. https://doi.org/10.1080/10715762.2018.1563689
Fujii T, Hayashi S, Hogg JC, Vincent R, Van Eeden SF (2001) Particulate matter induces cytokine expression in human bronchial epithelial cells. Am J Resp Cell Mol 25:265–271. https://doi.org/10.1165/ajrcmb.25.3.4445
Yu Q, Fu G, Lin H, Zhao Q, Liu Y, Zhou Y, Shi Y, Zhang L, Wang Z, Zhang Z (2020) Influence of silica particles on mucociliary structure and MUC5B expression in airways of C57BL/6 mice. Exp Lung Res 46:217–225. https://doi.org/10.1080/01902148.2020.1762804
Wolters PJ, Collard HR, Jones KD (2014) Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol-Mech 9:157–179. https://doi.org/10.1146/annurev-pathol-012513-104706
Lasky JA, Tonthat B, Liu J-y, Friedman M, Brody AR (1998) Upregulation of the PDGF-alpha receptor precedes asbestos-induced lung fibrosis in rats. Am J Resp Crit Care 157:1652–1657. https://doi.org/10.1164/ajrccm.157.5.9704116
Arcangeli G, Cupelli V, Giuliano G (2001) Effects of silica on human lung fibroblast in culture. Sci Total Environ 270:135–139. https://doi.org/10.1016/S0048-9697(00)00781-6
Xu YD, Hua J, Mui A, O’Connor R, Grotendorst G, Khalil N (2003) Release of biologically active TGF-β1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol-Lung C 285:L527–L539. https://doi.org/10.1152/ajplung.00298.2002
Scotton CJ, Chambers RC (2007) Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest 132:1311–1321. https://doi.org/10.1378/chest.06-2568
Hetzel M, Bachem M, Anders D, Trischler G, Faehling M (2005) Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung 183:225–237. https://doi.org/10.1007/s00408-004-2534-z
Srivastava KD, Rom WN, Jagirdar J, Yie T-A, Gordon T, Tchou-Wong K-M (2002) Crucial role of interleukin-1 β and nitric oxide synthase in silica-induced inflammation and apoptosis in mice. Am J Resp Crit Care 165:527–533. https://doi.org/10.1164/ajrccm.165.4.2106009
Sime PJ, Marr RA, Gauldie D, Xing Z, Hewlett BR, Graham FL, Gauldie J (1998) Transfer of tumor necrosis factor-α to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-β1 and myofibroblasts. Am J Pathol 153:825–832. https://doi.org/10.1016/S0002-9440(10)65624-6
Bonner JC (2002) The epidermal growth factor receptor at the crossroads of airway remodeling. Am J Physiol-Lung C 283:L528–L530. https://doi.org/10.1152/ajplung.00126.2002
Howard J, Huang A, Li Z, Tufekci Z, Zdimal V, van der Westhuizen H-M, von Delft A, Price A, Fridman L, Tang L-H (2021) An evidence review of face masks against COVID-19. P Natl Acad Sci USA 118:e2014564118. https://doi.org/10.1073/pnas.2014564118
Arellano-Cotrina JJ, Marengo-Coronel N, Atoche-Socola KJ, Peña-Soto C, Arriola-Guillén LE (2021) Effectiveness and recommendations for the use of dental masks in the prevention of COVID-19: a literature review. Disaster Med Public 15:e43–e48. https://doi.org/10.1017/dmp.2020.255
Becker K, Gurzawska-Comis K, Brunello G, Klinge B (2021) Summary of European guidelines on infection control and prevention during COVID-19 pandemic. Clin Oral Implan Res 32:353–381. https://doi.org/10.1111/clr.13784
Lonnroth E-C, Ruyter IE (2002) Permeability of medical gloves to mono- and dimethacrylate monomers in dental restorative materials. Int J Occup Saf Ergo 8:497–509. https://doi.org/10.1080/10803548.2002.11076540
Smith PB, Agostini G, Mitchell JC (2020) A scoping review of surgical masks and N95 filtering facepiece respirators: learning from the past to guide the future of dentistry. Safety Sci 131:104920. https://doi.org/10.1016/j.ssci.2020.104920
Roberge RJ (2016) Face shields for infection control: a review. J Occup Environ Hyg 13:239–246. https://doi.org/10.1080/15459624.2015.1095302
Remington WD, Ott BC, Hartka TR (2022) Effectiveness of barrier devices, high-volume evacuators, and extraoral suction devices on reducing dental aerosols for the dental operator: a pilot study. J Am Dent Assoc 153:309–318. e1. https://doi.org/10.1016/j.adaj.2021.08.011
Harrel SK, Molinari J (2004) Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc 135:429–437. https://doi.org/10.14219/jada.archive.2004.0207
Li X, Mak CM, Ma KW, Wong HM (2021) How the high-volume evacuation alters the flow-field and particle removal characteristics in the mock-up dental clinic. Build Environ 205:108225. https://doi.org/10.1016/j.buildenv.2021.108225
Chavis SE, Hines SE, Dyalram D, Wilken NC, Dalby RN (2021) Can extraoral suction units minimize droplet spatter during a simulated dental procedure? J Am Dent Assoc 152:157–165. https://doi.org/10.1016/j.adaj.2020.10.010
Senpuku H, Fukumoto M, Uchiyama T, Taguchi C, Suzuki I, Arikawa K (2021) Effects of extraoral suction on droplets and aerosols for infection control practices. Dent J 9:80. https://doi.org/10.3390/dj9070080
Suwandi T, Nursolihati V, Sundjojo M, Widyarman AS (2022) The efficacy of high-volume evacuators and extraoral vacuum aspirators in reducing aerosol and droplet in ultrasonic scaling procedures during the COVID-19 pandemic. Eur J Dent. https://doi.org/10.1055/s-0041-1739448
Zhao B, An N, Chen C (2021) Using an air purifier as a supplementary protective measure in dental clinics during the coronavirus disease 2019 (COVID-19) pandemic. Infect Cont Hosp Ep 42:493–493. https://doi.org/10.1017/ice.2020.292
Mohamed N, Yusof NA, Ali WNSW, Ismail NA (2018) The effectiveness of the X-Dent Box (dust collector box) in collecting trimming dust from different types of dental prostheses materials in Universiti Sains Islam Malaysia (USIM) dental laboratory. AIP Conf Proc 2030:020299. https://doi.org/10.1063/1.5066940
Srivastava A, Andersen MR, Alshehri AM, Lara B, Bashiri R, Li G, Chambers MS (2021) Effectiveness of a chairside acrylic adjustment cabinet in reducing dental acrylic debris and aerosols. J Prosthodont. https://doi.org/10.1111/jopr.13463
Yates DH, Perret JL, Davidson M, Miles SE, Musk A (2021) Dust diseases in modern Australia: a discussion of the new TSANZ position statement on respiratory surveillance. Med J Australia 215:13–15. e1. https://doi.org/10.5694/mja2.51097
Gamio L (2020) The workers who face the greatest coronavirus risk. The New York Times. https://www.nytimes.com/interactive/2020/03/15/business/economy/coronavirus-worker-risk.html Accessed 21 December 2022
Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B (2020) Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci 12:1–6. https://doi.org/10.1038/s41368-020-0075-9
Campus G, Betancourt MD, Cagetti MG et al (2021) The COVID-19 pandemic and its global effects on dental practice. An international survey. J Dent 114:103749. https://doi.org/10.1016/j.jdent.2021.103749
Huh D, Matthews BD, Mammoto A et al (2010) Reconstituting organ-level lung functions on a chip. Science 328:1662–1668. https://doi.org/10.1126/science.1188302
Funding
The work was financially supported by Science and Technology Project of Jilin Province Financial Department (JCSZ2019378-7) (LF); General Program of Natural Science Foundation of Jilin Province (20200201592JC) (LF); and Health Department Research Projects of Jilin Province (2019J023) (LF).
Author information
Authors and Affiliations
Contributions
Jiaxin Ding and Junxuan Li reviewed the literatures and contributed to manuscript drafting; Junnan Qi was responsible for the revision of the manuscript; Li Fu contributed to study design and manuscript supervision; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, J., Li, J., Qi, J. et al. Characterization of dental dust particles and their pathogenicity to respiratory system: a narrative review. Clin Oral Invest 27, 1815–1829 (2023). https://doi.org/10.1007/s00784-023-04910-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-04910-w