Abstract
Objectives
This study aimed to investigate whether combined exposure to starch and sucrose modifies the activity of carbonic anhydrase VI (CA VI) in saliva (Study 1) and biofilm (Study 2) of children with early childhood caries (ECC).
Material and methods
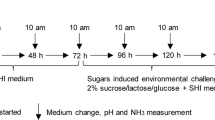
For Study 1 and Study 2, respectively, 54 and 46 preschoolers aged 4 to 5 were allocated into two groups: caries-free (CF) and with ECC. Children were exposed to rinses with sucrose, starch, and sucrose plus starch solutions. CA VI activity, pH, and buffering capacity (BC) were evaluated in saliva and biofilm.
Results
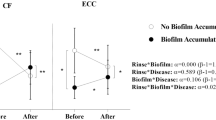
In Study 1, a significant reduction in saliva pH was observed after sucrose and sucrose plus starch rinses. CA VI activity was influenced by ECC independently of the type of carbohydrate to which children were exposed. CA VI activity was higher in children with ECC; however, after rinses, CA VI activity was reduced. In Study 2, biofilm pH and BC were reduced after rinses with sucrose and sucrose plus starch. CA VI activity was significantly high before rinse in ECC group when compared with CF group; however, no difference was observed between groups after rinses.
Conclusions
In saliva, exposure to starch and sucrose (isolated or combined) induced a reduction in CA VI activity in children with ECC. In biofilm, the combination of starch and sucrose did not modify CA VI activity in ECC children.
Clinical relevance
The responsivity of the CA VI reflects directly in important parameters related to the pH maintenance on the oral cavity.




Similar content being viewed by others
References
Tinanoff N, Baez RJ, Diaz Guillory C, Donly KJ, Feldens CA, McGrath C, Phantumvanit P, Pitts NB, Seow WK, Sharkov N, Songpaisan Y, Twetman S (2019) Early childhood caries epidemiology, aetiology, risk assessment, societal burden, management, education, and policy: Global perspective. Int J Paediatr Dent 29(3):238–248
Moynihan PJ, Kelly SA (2014) Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. J Dent Res 93(1):8–18
Sheiham A, James WPT (2015) Diet and dental caries: the pivotal role of free sugars reemphasized. J Dent Res 94(10):1341–1347
Peres MA, Sheiham A, Liu P, Demarco FF, Silva AER, Assuncao MC, Menezes AM, Barros FC, Peres KG (2016) Sugar consumption and changes in dental caries from childhood to adolescence. J Dent Res 95(4):388–394
Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA (2006) The role of sucrose in cariogenic dental biofilm formation--new insight. J Dent Res 85(10):878–887
Cai JN, Jung JE, Dang MH, Kim MA, Yi HK, Jeon JG (2016) Functional relationship between sucrose and a cariogenic biofilm formation. PLoS One 11(6):e0157184
Hwang G, Liu Y, Kim D, Sun V, Aviles-Reyes A, Kajfasz JK, Lemos JA, Koo H (2016) Simultaneous spatiotemporal mapping of in situ pH and bacterial activity within an intact 3D microcolony structure. Sci Rep 6:32841
Ribeiro C, Tabchoury C, Del Bel CA, Tenuta L, Rosalen P, Cury J (2005) Effect of starch on the cariogenic potential of sucrose. Br J Nutr 94(1):44–50
Aires CP, Del Bel Cury AA, Tenuta LM, Klein MI, Koo H, Duarte S, Cury JA (2008) Effect of starch and sucrose on dental biofilm formation and on root dentine demineralization. Caries Res 42(5):380–386
Botelho JN, Villegas-Salinas M, Troncoso-Gajardo P, Giacaman RA, Cury JA (2016) Enamel and dentine demineralization by a combination of starch and sucrose in a biofilm - caries model. Braz Oral Res Brazil 30(1)
Campain AC, Morgan MV, Evans RW, Ugoni A, Adams GG, Conn JA, Watson MJ (2003) Sugar-starch combinations in food and the relationship to dental caries in low-risk adolescents. Eur J Oral Sci 111(4):316–325
Halvorsrud K, Lewney J, Craig D, Moynihan PJ (2019) Effects of starch on oral health: systematic review to inform WHO guideline. J Dent Res 98(1):46–53
Guo L, Shi W (2013) Salivary biomarkers for caries risk assessment. J Calif Dent Assoc 41(2):107–109 112-8
Gao X, Jiang S, Koh D, Hsu CY (2016) Salivary biomarkers for dental caries. Periodontol 2000 70(1):128–141
Lips A, Antunes LS, Antunes LA, Pintor AVB, Dos Santos DAB, Bachinski R, Küchler EC, Alves GG (2017) Salivary protein polymorphisms and risk of dental caries: a systematic review. Braz Oral Res Brazil 31:e41
Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, Tagami J, Twetman S, Tsakos G, Ismail A (2017) Dental caries. Nat Rev Dis Primers 3:17030
Belda-Ferre P, Williamson J, Simón-Soro Á, Artacho A, Jensen ON, Mira A (2015) The human oral metaproteome reveals potential biomarkers for caries disease. Proteomics 15(20):3497–3507
Ao S, Sun X, Shi X, Huang X, Chen F, Zheng S (2017) Longitudinal investigation of salivary proteomic profiles in the development of early childhood caries. J Dent 61:21–27
Wang K, Zhou X, Li W, Zhang L (2019) Human salivary proteins and their peptidomimetics: values of function, early diagnosis, and therapeutic potential in combating dental caries. Arch Oral Biol 99:31–42
Kimoto M, Kishino M, Yura Y, Ogawa Y (2006) A role of salivary carbonic anhydrase VI in dental plaque. Arch Oral Biol 51(2):117–122
Esberg A, Haworth S, Brunius C, Lif Holgerson P, Johansson I (2019) Carbonic anhydrase 6 Gene variation influences oral microbiota composition and caries risk in Swedish adolescents. Sci Rep 9(1):452
Kivela J, Parkkila S, Parkkila AK, Leinonen J, Rajaniemi H (1999) Salivary carbonic anhydrase isoenzyme VI. J Physiol 520(2):315–320
Leinonen J, Kivela J, Parkkila S, Parkkila AK, Rajaniemi H (1999) Salivary carbonic anhydrase isoenzyme VI is located in the human enamel pellicle. Caries Res 33(3):185–190
Daniele de Cassia Rodrigues Picco, Lopes LM, Rocha Marques M, Line SRPP, Parisotto TM, Nobre Dos Santos M. Children with a higher activity of carbonic anhydrase vi in saliva are more likely to develop dental caries. Caries Res 2017;51(4):394-401.
Picco DCR, Marangoni-Lopes L, Parisotto TM, Mattos-Graner R, Nobre dos Santos M (2019) Activity of carbonic anhydrase VI is higher in dental biofilm of children with caries. Int J Mol Sci 20(11):2673
Borghi GN, Rodrigues LP, Lopes LM, Parisotto TM, Steiner-Oliveira C, Nobre-Dos-Santos M (2017) Relationship among alpha amylase and carbonic anhydrase VI in saliva, visible biofilm, and early childhood caries: a longitudinal study. Int J Paediatr Dent 27(3):174–182
Frasseto F, Parisotto TM, Peres RCR, Marques MR, Line SRP (2012) Nobre Dos Santos M. Relationship among salivary carbonic anhydrase VI activity and flow rate, biofilm pH and caries in primary dentition. Caries Res 46(3):194–200
Bowen WH, Koo H (2011) Biology of streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res 45(1):69–86
Assaf AV, de Castro Meneghim M, Zanin L, Tengan C, Pereira AC (2006) Effect of different diagnostic thresholds on dental caries calibration - a 12 month evaluation. Community Dent Oral Epidemiol 34(3):213–219
World Health Organization (2013) Oral health surveys: basic methods, 5th edn, Geneva, p 125
Alaluusua S, Malmivirta R (1994) Early plaque accumulation - a sign for caries risk in young children. Community Dent Oral Epidemiol 22(5 Pt 1):273–276
Dawes C (1972) Circadian rhythms in human salivary flow rate and composition. J Physiol 220(3):529–545
Parkkila S, Parkkila AK, Rajaniemi H (1995) Circadian periodicity in salivary carbonic anhydrase VI. Acta Physiol Scand 154(2):205–211
Kivela J, Parkkila S, Waheed A, Parkkila AK, Sly WS, Rajaniemi H (1997) Secretory carbonic anhydrase isoenzyme (CA VI) in human serum. Clin Chem 43(12):2318–2322
Bardow A, Madsen J, Nauntofte B (2000) The bicarbonate concentration in human saliva does not exceed the plasma level under normal physiological conditions. Clin Oral Investig 4(4):245–253
Denepitiya L, Kleinberg I (1982) A comparison of the microbial compositions of pooled human dental plaque and salivary sediment. Arch Oral Biol 27(9):739–745
Lecomte P, Dawes C (1987) The influence of salivary flow rate on diffusion of potassium chloride from artificial plaque at different sites in the mouth. J Dent Res 66(11):1614–1618
Higham SM, Edgar WM (1989) Effects of Parafilm and cheese chewing on human dental plaque pH and metabolism. Caries Res 23(1):42–48
Shellis RP, Dibdin GH (1988) Analysis of the buffering systems in dental plaque. J Dent Res 67(2):438–446
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Kotwica J, Ciuk MA, Joachimiak E, Rowinski S, Cymborowski B, Bebas P (2006) Carbonic anhydrase activity in the vas deferens of the cotton leafworm - Spodoptera littoralis (Lepidoptera: Noctuidae) controlled by circadian clock. J Physiol Pharmacol 57(Suppl 8):107–123
Aidar M, Marques R, Valjakka J, Mononen N, Lehtimäki T, Parkkila S, de Souza AP, Line P (2013) Effect of genetic polymorphisms in CA6 gene on the expression and catalytic activity of human salivary carbonic anhydrase VI. Caries Res 47(5):414–420
Collins TJ (2007) ImageJ for microscopy. Biotechniques. 43(1 Suppl):25–30
Samal M, Karny M, Benali H, Backfrieder W, Todd-Pokropek A, Bergmann H (1999) Experimental comparison of data transformation procedures for analysis of principal components. Phys Med Biol 44(11):2821–2834
American Academy of Pediatric Dentistry (2014) Caries-risk assessment and management for infants, children, and adolescents. Pediatr Dent 36(special issue):127–134
Duggal MS, Toumba KJ, Amaechi BT, Kowash MB, Higham SM (2001) Enamel demineralization in situ with various frequencies of carbohydrate consumption with and without fluoride toothpaste. J Dent Res 80(8):1721–1724
Kim SW (2012) Environmental, maternal, and child factors which contribute to early childhood caries: a unifying conceptual model. Int J Paediatr Dent 22(3):157–168
Marsh PD. In sickness and in health-what does the oral microbiome mean to us? An ecological perspective Adv Dent Res 2018;29(1):60-65.
Zeng Y (2011) The functional consequences of relative substrate specificity in complex biochemical systems. Front Genet 2:65
Buonanno M, Di Fiore A, Langella E, D'Ambrosio K, Supuran CT, Monti SM, De Simone G (2018) The crystal structure of a hCA VII variant provides insights into the molecular determinants responsible for its catalytic behavior. Int J Mol Sci 19(6):E1571
Ding HM, Shao L, Liu RJ, Xiao QG, Chen JF (2005) Silica nanotubes for lysozyme immobilization. J Colloid Interface Sci 290(1):102–106
Fears KP, Latour RA (2009) Assessing the influence of adsorbed-state conformation on the bioactivity of adsorbed enzyme layers. Langmuir 25(24):13926–13933
Margolis HC, Duckworth JH, Moreno EC (1988) Composition and buffer capacity of pooled starved plaque fluid from caries-free and caries-susceptible individuals. J Dent Res 67(12):1476–1482
Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR 3rd, Heydorn A, Koo H (2012) The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog 8(4):e1002623
Mormann JE, Muhlemann HR (1981) Oral starch degradation and its influence on acid production in human dental plaque. Caries Res 15(2):166–175
Lingström P, Holm J, Birkhed D, Björck I (1989) Effects of variously processed starch on pH of human dental plaque. Scand J Dent Res 97(5):392–400
Linke HA, Birkenfeld LH (1999) Clearance and metabolism of starch foods in the oral cavity. Ann Nutr Metab 43(3):131–139
Kashket S, Zhang J, Van Houte J (1996) Accumulation of fermentable sugars and metabolic acids in food particles that become entrapped on the dentition. J Dent Res 75(11):1885–1891
Gupta P, Gupta N, Pawar AP, Birajdar SS, Natt AS, Singh HP (2013) Role of sugar and sugar substitutes in dental caries: A Review. ISRN Dent 2013:519421
Kumar A, Hedge R, Dixit U (2011) Role of plaque in the clearance of salivary sucrose and its influence on salivary pH. J Indian Soc Pedod Prev Dent 29(4):310–314
Dawes C (2008) Salivary flow patterns and the health of hard and soft oral tissues. J Am Dent Assoc 139(Suppl):18S–24S
Dawes C, Watanabe S (1987) The effect of taste adaptation on salivary flow rate and salivary sugar clearance. J Dent Res 66(3):740–744
Lingström P, van Houte J, Kashket S (2000) Food starches and dental caries. Crit Rev Oral Biol Med 11(3):366–380
Nikitkova AE, Haase EM, Scannapieco FA (2013) Taking the starch out of oral biofilm formation: molecular basis and functional significance of salivary alpha-amylase binding to oral streptococci. Appl Environ Microbiol 79(2):416–423
Fisher SZ, Govindasamy L, Tu C, Agbandje-McKenna M, Silverman DN, Rajaniemi HJ, McKenna R (2006) Structure of human salivary alpha-amylase crystallized in a C-centered monoclinic space group. Acta Crystallogr Sect F Struct Biol Cryst Commun 62(Pt 2):88–93
Joubert M, Septier C, Brignot H, Salles C, Panouillé M, Feron G, Tournier C (2017) Chewing bread: impact on alpha-amylase secretion and oral digestion. Food Funct 8(2):607–614
Vandooren J, Geurts N, Martens E, Van den Steen PE, Opdenakker G (2013) Zymography methods for visualizing hydrolytic enzymes. Nat Methods 10(3):211–220
Acknowledgments
This study was based on a thesis submitted to the Piracicaba Dental School-UNICAMP/Brazil, to obtain a Ph.D. degree in Odontology. The authors thank CNPq (National Counsel of Technological and Scientific Development) for awarding a Ph.D. grant to the first author. We especially thank all preschoolers and their parents for their valuable participation in this study.
Funding
This work was supported by the FAPESP [grant number 2017/17630-8].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted under the guidelines of the Declaration of Helsinki. The Research Ethics Committee of the Piracicaba Dental School—University of Campinas approved this research (CAAE: 70777517.9.0000.5418).
Informed consent
Parents or guardians who agreed with the participation of their children in this research signed a free informed consent. The selected preschoolers also gave their permission to take part in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 96 kb)
Rights and permissions
About this article
Cite this article
de Sousa, E.T., Lima-Holanda, A.T., Sales, L.S. et al. Combined effect of starch and sucrose on carbonic anhydrase VI activity in saliva and biofilm of children with early childhood caries. Exposure to starch and sucrose alters carbonic anhydrase VI activity in saliva and biofilm. Clin Oral Invest 25, 2555–2568 (2021). https://doi.org/10.1007/s00784-020-03567-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03567-z