Abstract
Objectives
Several studies have recently demonstrated that only marginal improvements in platelet and leukocyte concentrations are achieved following standard injectable platelet-rich fibrin (i-PRF) protocols. Due to these previous findings, a novel harvesting technique was recently developed to collect higher concentrations of platelets/leukocytes specifically from the buffy coat layer (C-PRF) following faster centrifugation protocols. The aim of this study was to investigate the regenerative properties and effects on growth factor release and cellular activity of PRF collected through this novel harvesting technique compared to standard i-PRF protocols.
Materials and methods
The upper 1-ml layer collected through standard i-PRF protocols at low centrifugation speeds was compared with 1 mL of C-PRF collected from the buffy coat layer following high centrifugation protocols (3000×g for 8 min on a horizontal centrifuge) to specifically concentrate cells within the platelet/leukocyte-rich buffy coat layer. Thereafter, the expression of seven different growth factors, including PDGF-AA, PDGF-AB, PDGF-BB, TGF-β1, VEGF, IGF-1, and EGF, was characterized for up to 10 days. Then, gingival fibroblast biocompatibility was investigated at 24 h (live/dead assay); migration was investigated at 24 h; proliferation was investigated at 1, 3, and 5 days; and the expression of PDGF and TGF-β was investigated at 3 days. Collagen 1 immunostaining was also quantified at 14 days.
Results
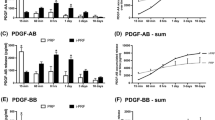
At all investigated time periods, a significant increase in growth factor release was observed in C-PRF. In particular, the release of PDGF-AA, TGF-β1, and EGF exhibited the highest increases when compared with that in i-PRF. While both i-PRF and C-PRF exhibited high biocompatibility and induced significantly higher fibroblast migration and proliferation when compared with that of the control tissue culture plastic group, C-PRF showed the greatest potential for cell migration and proliferation. Furthermore, C-PRF induced significantly higher mRNA levels of TGF-β and PDGF levels at 3 days and greater collagen 1 staining when compared with induced by i-PRF.
Conclusions
In the present study, it was found that C-PRF collected specifically from the buffy coat layer following higher centrifugation protocols exhibited an up to a threefold increase in growth factor release when compared with that exhibited by standard i-PRF. This significantly promoted higher gingival fibroblast migration, proliferation, gene expression, and collagen I synthesis.
Clinical relevance
The findings of the present study demonstrate that a more potent formulation of liquid platelet concentrate than that obtained from the upper plasma layer following a short and slow centrifugation protocol (i-PRF protocol) can be obtained for clinical use by specifically harvesting cells in the platelet- and leukocyte-rich buffy coat layer following an 8-min 3000×g centrifugation protocol (C-PRF protocol).






Similar content being viewed by others
References
Chow TW, McIntire LV, Peterson DM (1983) Importance of plasma fibronectin in determining PFP and PRP clot mechanical properties. Thromb Res 29:243–248
Delaini F, Poggi A, Donati MB (1982) Enhanced affinity for arachidonic acid in platelet-rich plasma from rats with Adriamycin-induced nephrotic syndrome. Thromb Haemost 48:260–262
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62:489–496
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:638–646
Cai YZ, Zhang C, Lin XJ (2015) Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: a meta-analysis. J Shoulder Elbow Surg 24:1852–1859. https://doi.org/10.1016/j.jse.2015.07.035
Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD (2016) Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy 32:495–505. https://doi.org/10.1016/j.arthro.2015.08.005
Singh B, Goldberg LJ (2016) Autologous platelet-rich plasma for the treatment of pattern hair loss. Am J Clin Dermatol 17:359–367. https://doi.org/10.1007/s40257-016-0196-2
Anfossi G, Trovati M, Mularoni E, Massucco P, Calcamuggi G, Emanuelli G (1989) Influence of propranolol on platelet aggregation and thromboxane B2 production from platelet-rich plasma and whole blood. Prostaglandins Leukot Essent Fat Acids 36:1–7
Fijnheer R, Pietersz RN, de Korte D, Gouwerok CW, Dekker WJ, Reesink HW, Roos D (1990) Platelet activation during preparation of platelet concentrates: a comparison of the platelet-rich plasma and the buffy coat methods. Transfusion 30:634–638
Choukroun J, Adda F, Schoeffler C, Vervelle A (2001) Une opportunité en paro-implantologie: le PRF. Implantodontie 42:e62
Toffler M (2014) Guided bone regeneration (GBR) using cortical bone pins in combination with leukocyte-and platelet-rich fibrin (L-PRF). Compendium of Continuing Educ Dentist 35:192–198
Lekovic V, Milinkovic I, Aleksic Z, Jankovic S, Stankovic P, Kenney E, Camargo P (2012) Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J Periodontal Res 47:409–417
Shivashankar VY, Johns DA, Vidyanath S, Sam G (2013) Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory periapical lesion. J Conserv Dent 16:261
Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL, Chandad F, Nacopoulos C, Simonpieri A, Aalam AA, Felice P, Sammartino G, Ghanaati S, Hernandez MA, Choukroun J (2017) Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig 21:1913–1927. https://doi.org/10.1007/s00784-017-2133-z
Medina-Porqueres I, Alvarez-Juarez P (2015) The efficacy of platelet-rich plasma injection in the management of hip osteoarthritis: a systematic review protocol. Musculoskelet Care 14:121–125. https://doi.org/10.1002/msc.1115
Salamanna F, Veronesi F, Maglio M, Della Bella E (2015) New and emerging strategies in platelet-rich plasma application in musculoskeletal regenerative procedures: general overview on still open questions and outlook. Biomed Res Int 2015:846045. https://doi.org/10.1155/2015/846045
Albanese A, Licata ME, Polizzi B, Campisi G (2013) Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing 10:23. https://doi.org/10.1186/1742-4933-10-23
Panda S, Doraiswamy J, Malaiappan S, Varghese SS, Del Fabbro M (2014) Additive effect of autologous platelet concentrates in treatment of intrabony defects: a systematic review and meta-analysis. J Investig Clin Dent 7:13–26. https://doi.org/10.1111/jicd.12117
Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, Choukroun J (2017) Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig 21:2619–2627. https://doi.org/10.1007/s00784-017-2063-9
Abd El Raouf M, Wang X, Miusi S, Chai J, Mohamed AbdEl-Aal AB, Nefissa Helmy MM, Ghanaati S, Choukroun J, Choukroun E, Zhang Y, Miron RJ (2017) Injectable-platelet rich fibrin using the low speed centrifugation concept improves cartilage regeneration when compared to platelet-rich plasma. Platelets. https://doi.org/10.1080/09537104.2017.1401058
Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ (2017) Behavior of gingival fibroblasts on titanium implant surfaces in combination with either injectable-PRF or PRP. Int J Mol Sci. https://doi.org/10.3390/ijms18020331
Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ (2018) Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 29:48–55. https://doi.org/10.1080/09537104.2017.1293807
Varela HA, Souza JCM, Nascimento RM, Araujo RF Jr, Vasconcelos RC, Cavalcante RS, Guedes PM, Araujo AA (2019) Injectable platelet rich fibrin: cell content, morphological, and protein characterization. Clin Oral Investig 23:1309–1318. https://doi.org/10.1007/s00784-018-2555-2
Miron RJ, Chai J, Zheng S, Feng M, Sculean A, Zhang Y (2019) A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A 107:2257–2271. https://doi.org/10.1002/jbm.a.36734
Wend S, Kubesch A, Orlowska A, Al-Maawi S, Zender N, Dias A, Miron RJ, Sader R, Booms P, Kirkpatrick CJ, Choukroun J, Ghanaati S (2017) Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J Mater Sci Mater Med 28:188. https://doi.org/10.1007/s10856-017-5992-6
Castro AB, Cortellini S, Temmerman A, Li X, Pinto N, Teughels W, Quirynen M (2019) Characterization of the leukocyte- and platelet-rich fibrin block: release of growth factors, cellular content, and structure. Int J Oral Maxillofac Implants 34:855–864. https://doi.org/10.11607/jomi.7275
Cortellini S, Castro AB, Temmerman A, Van Dessel J, Pinto N, Jacobs R, Quirynen M (2018) Leucocyte- and platelet-rich fibrin block for bone augmentation procedure: a proof-of-concept study. J Clin Periodontol 45:624–634. https://doi.org/10.1111/jcpe.12877
Miron RJ, Chai J, Zhang P, Li Y, Wang Y, Mourao C, Sculean A, Fujioka Kobayashi M, Zhang Y (2019) A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clin Oral Investig. https://doi.org/10.1007/s00784-019-03147-w
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 20:2353–2360. https://doi.org/10.1007/s00784-016-1719-1
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J (2016) Optimized platelet rich fibrin with the low speed concept: growth factor release, biocompatibility and cellular response. J Periodontol. https://doi.org/10.1902/jop.2016.160443
Bielecki T, Dohan Ehrenfest DM, Everts PA, Wiczkowski A (2012) The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr Pharm Biotechnol 13:1153–1162
Martin P (1997) Wound healing--aiming for perfect skin regeneration. Science 276:75–81
Barrick B, Campbell EJ, Owen CA (1999) Leukocyte proteinases in wound healing: roles in physiologic and pathologic processes. Wound Repair Regen 7:410–422
Miron RJ, Xu H, Chai J, Wang J, Zheng S, Feng M, Zhang X, Wei Y, Chen Y, Mourao C, Sculean A, Zhang Y (2019) Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~700 g) and low (~200 g) relative centrifugation forces. Clin Oral Investig 24:1171–1182. https://doi.org/10.1007/s00784-019-02981-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Richard J Miron holds intellectual property on concentrated PRF. All other authors declare no conflict of interest.
Ethical approval
No ethical approval was required for this study, as the human samples were not identified in this study.
Informed consent
For this type of study, informed consent was not required, as no human or animal subjects were utilized.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PNG 5805 kb)
Rights and permissions
About this article
Cite this article
Fujioka-Kobayashi, M., Katagiri, H., Kono, M. et al. Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared with traditional injectable (i-PRF) protocols. Clin Oral Invest 24, 4373–4383 (2020). https://doi.org/10.1007/s00784-020-03303-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03303-7