Abstract
Objectives
To assess how to control, detect, and treat secondary caries. This review serves to inform a joint ORCA/EFCD consensus process.
Methods
Systematic and non-systematic reviews were performed or consulted and narratively synthesized.
Results
Secondary (or recurrent) caries is defined as a lesion associated with restorations or sealants. While the restorative material itself has some influence on secondary caries, further factors like the presence and size of restoration gaps, patients’ caries risk, and the placing dentist’s experience seem more relevant. Current detection methods for secondary caries are only sparsely validated and likely prone for the risk of over-detection. In many patients, it might be prudent to prioritize specific detection methods to avoid invasive overtreatment. Detected secondary caries can be managed either by repair of the defective part of the restoration or its complete replacement.
Conclusions
There is sparse data towards the nature of secondary caries and how to control, detect, and treat it.
Clinical significance
Despite often claimed to be a major complication of restorations, there is surprisingly little data on secondary caries. Longer-term studies may be needed to identify differences in secondary caries risk between materials and to identify characteristic features of progressive lesions (i.e., those in need of treatment).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary (or recurrent) caries [1] has been defined as “lesions at the margins of existing restorations” [2] or “caries associated with restorations or sealants” (CARS). Secondary caries is a complex, multifactorial process, interweaving the various causes of “conventional” caries with the specific characteristics of the restoration and restorative material involved, i.e., secondary caries pathogenesis follows the same concept for any other caries lesions, involving demineralization and, in case of dentin secondary caries, enzymatic dissolution of the organic component, but is modified by the presence of a restoration or sealant margin. This specific modification and its relevance is a matter of active debate.
Secondary caries may be (1) causally associated with a defective restoration (mainly via gaps between the restoration and the tooth allowing acidic fluids or biofilm to enter the interface) or (2) causally associated with an intact restoration (e.g., via a lower buffering capacity of the restoration compared with the tooth hard tissue) or (3) not causally associated with the restoration at all, but mere primary caries adjacent to existing restorations (mainly when the caries process has not been sufficiently addressed on a patient level and the surface next to the restoration becomes carious as a result of this ongoing caries activity) [3,4,5]. For all three cases, though, the known factors relevant for caries development (presence of a cariogenic biofilm, supply with fermentable carbohydrates, imbalance in mineral loss, and loss of dental hard tissue) are required.
The result of these different pathogenic pathways may be demineralization on the tooth surface, as typically occurs in primary carious lesions, as well as along the interface (Fig. 1), with a surface and a so-called wall lesion [5,6,7,8]. There is some debate around the nature and size of a restoration defect required to allow formation of such wall lesions. A number of in vitro studies, using various secondary caries models, have been performed, yielding threshold gap sizes of the defect between 60 and 1000 μm [9, 10]. In situ studies confirmed the range of these gap sizes. More recent studies found masticatory loading on the restoration to enhance wall lesion formation, enabling such formation also along relatively small gap sizes, possibly as the cyclic loading facilitates fluid (and dysbiotic biofilm) penetration along the interface [11,12,13]. Findings from in vivo studies, however, could not necessarily confirm such threshold gaps sizes or effects of loading, mainly due to methodological limitations [5]. The presence of gaps may be the result of an imperfect initial placement of the restoration, for example by non-compensated polymerization shrinkage or insufficient light-curing of the material (with a subsequent wash-out of un-cured components) [14]. Long-term defects and gaps may also form by hydrolytic degradation of the hybrid layer and hence the interface in case of adhesive (resin-based) restorations [15, 16].
Can we control secondary caries risk?
A number of factors have been evaluated for their association with secondary caries risk. If they were to be controlled, the risk for secondary caries could be controlled, too.
The most frequent ones, synthesized in a recent review [17], are the following:
-
Surface location. The vast majority of secondary carious lesions (up to 90%) are found at the gingival margin of restorations, regardless of the restoration material [18, 19]. It was further speculated that “deeper” proximal restorations, i.e., those extending to the cementum-enamel junction or beyond, show higher risks of secondary caries, as the dental substrate is not enamel, but cementum and dentin in this case [20], but also as restoration placement may be more challenging for these deeper restorations (see above). The sparse data available does not necessarily confirm this hypothesis, though [21]. Moreover, most data are collected from posterior restorations; in anterior regions, secondary caries is less likely to occur [22].
-
Patients’ caries risk/susceptibility. The risk of restoration failure due to secondary caries is significantly increased in high-risk caries patients compared with low-risk ones [23, 24]. Such patients usually also show limited adherence, which needs to be considered during diagnostic and treatment decisions.
-
Patients’ age. It remains unclear if age mainly serves as proxy for caries susceptibility (being higher in very young and the older patient) or if there is truly an association between secondary caries susceptibility and age. It is also conceivable that the placement of restorations will come with different challenges in different age groups, which may be reflected in the risk of secondary caries, as discussed.
-
Socioeconomic status. While the socioeconomic status may, indirectly via behavioral traits, affect caries susceptibility (see above), it remains unclear if it is also associated with “different” restorations (placed differently, using different materials or protocols). There is evidence that socioeconomic status is associated with longevity of restorations and secondary caries [25, 26].
-
Operator skill variability. As discussed, secondary caries is partially associated with the quality of the restoration placed. Besides the patient as one main factor, the operator is often seen as the second main factor impacting on restoration longevity and risk of secondary caries (both being possibly more relevant than the specific restorative protocol or material, see below). Operator’s experience and care during placing the restoration will affect its integrity and long-term survival. Practice-based studies demonstrate the impact of operators on restoration longevity [25,26,27,28]. Operator skills also affect how detections are handled and transformed into treatment decisions (see below).
-
Detection methods and criteria. As discussed below, detecting secondary caries is a challenge. Most detection methods and criteria have limited accuracy [29]. While, of course, detection methods and criteria will not affect the “development” of secondary caries, they will determine how often a finding will be defined as secondary caries or not. Detection methods and criteria will, for example, impact on the reported incidence rates of secondary caries. In this sense, it is unclear at present if the high incidence rates reported from long-term data, mainly stemming from non-controlled cohorts, are fully ascribable to secondary caries. In many instances, discolorations or clinically irrelevant imperfections may have been identified as secondary caries [19, 30, 31]. At present, there is sparse evidence demonstrating what kind of characteristics an identified defect needs to progress into a state where care is undoubtedly needed, or to remain in the status quo in the long term, possibly never progressing and hence never needing care [32].
Do different materials affect secondary caries risk?
While a patient’s caries risk or susceptibility seems to be the most relevant factor for secondary caries development, the relevance of the restorative material has been evaluated most frequently. A large number of studies comparing different materials have assessed secondary caries, either in vitro, in situ, or clinically.
In vitro studies offer the opportunity to evaluate different factors under cariogenic environment (chemical demineralization or biofilm model) with direct correlation to the histological changes. The effect of an interfacial gap between the restoration and tooth structure was evaluated by a large number of studies, with gap size and the used filling materials being the mostly tested factors. The available studies found no significant difference between various resin materials on the mineral loss of both surface and wall lesions. However, amalgam and fluoride-containing materials (e.g., glass ionomers) were able to reduce secondary caries progression in some studies [11, 33, 34]. It is unclear how these findings relate to clinical settings, e.g., patients using fluoridated toothpaste, etc. Generally, large methodological heterogeneity applies to these in vitro studies, with various restorative protocols, methods to simulate caries, and techniques to evaluate mineral loss having been used [35,36,37].
In situ studies, where samples composed of dental hard tissues and restorative materials are worn in fixed or removable dental appliances for some days or weeks, have the advantage of testing different materials under natural oral environment. A recent systematic review could not identify significant and consistent differences in secondary caries development next to different restorations [38]. The authors of the review included nine studies (132 patients, 8 materials) and performed network meta-analysis for synthesizing the results. They found that any material rankings come with uncertainty and identified ambiguity or even contradiction between studies. A wide range of materials and materials combinations (composites-adhesive combination, glass ionomer, resin-modified glass ionomer, amalgam) was used; the majority of studies compared 2–3 materials [38]. The specimens were placed in three positions: palatal, buccal, and, rarely, proximal. A split-mouth design was the most frequently used; in case of fluoride-releasing materials, the cross-over design was also frequent to avoid a carry-over effect. Also, different substrates (bovine or human enamel and/or dentin) and patients (low vs. high caries) were found [38]. The progression of secondary caries in in situ studies was remarkably higher than in a clinical situation, mainly by intentionally accelerating lesion progression by creating highly cariogenic environment (e.g., covering the specimens with a mesh to allow a non-disturbed development of the biofilm, frequent application of sucrose) [38, 39]. Overall, there are doubts as to the validity, consistency, and transferability of the in situ evidence to clinical settings. Especially the results of single in situ studies seem to be closely related to methodological decisions rather than replicable differences between materials.
Clinical studies compared a range of materials for their risk of secondary caries. The former “standard” dental material, amalgam, seems to come with lower risk of secondary caries, especially in high caries risk patients, as confirmed by randomized controlled studies [40, 41]. Notably, though, and considering the phase-down or phase-out in many countries worldwide which have signed the Minamata agreement [42], more recent studies assessed amalgam alternatives, like incrementally placed composites, bulk-fill composites, or glass ionomers (GI), for their risk of secondary caries [43]. Surprisingly, only a very limited body of evidence, at least from randomized controlled trials, is available for this comparison. Composites show good performance if placed in low-risk patients, and also for rather extended cavities, without a clear difference between incremental and bulk placement. Notable, many studies included in the available reviews did not place “bulk-fill composites” in bulk, and from those which did, there is no clear indication that such bulk placement comes with increased risk of failure (possibly, though, as the follow-up period of these studies was low, below 5 years) [43]. GIs show similar performance with regard to secondary caries when compared with composites but may come with higher risk of fracture in extended cavities. Notably, the most recent developments of GIs have only very sparsely been assessed clinically. Some countries have adopted GIs as the standard amalgam alternative given its applicability and placement costs [44].
Overall and based on randomized controlled trials, the impact of the restorative material on secondary caries risk seems to be limited. Notably, though, the follow-up period of these trials was short, and most were performed in low-risk patients under controlled (university) settings. Hence, the overall number of lesions which developed was low. Longer-term practice-based studies reported on higher number of developed secondary caries lesions, while the overall conclusions of these studies point into a similar direction [45, 46].
In summary, patient- and operator-level factors seem to be decisive for controlling secondary caries. Especially the caries risk or susceptibility of a patient is associated with secondary caries development.
Detecting secondary carious lesions
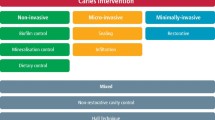
Early detection of secondary caries may allow provision of less invasive treatment options like re-sealing or repair instead of complete removal and replacement of restorations (see below). Detection of secondary caries can either be highly sensitive, detecting nearly all lesions, but coming with a concomitant high risk of false-positive diagnoses, or highly specific, avoiding such false-positive diagnoses but consequentially missing many lesions. There will always be, to some degree, a trade-off between sensitivity and specificity and the resulting over- and under-detection or over- and under-treatment [29].
A variety of methods are available to detect secondary caries, including visual, tactile, radiographic, laser fluorescence, and quantitative light-induced fluorescence assessments [47]. A recent review [29] summarized the available evidence on these detection methods and their accuracy. For this review, clinical or in vitro studies investigating the accuracy of these five detection methods on natural or artificially induced secondary lesions were included. A diagnostic accuracy meta-analysis was performed, and sensitivity, specificity, and positive and negative likelihood ratios were estimated. From 1179 screened studies, 23 were included; all but two were performed in vitro and all had high risk of bias. Lesions were mainly assessed in permanent teeth (n = 21); two studies assessed primary teeth. Lesions were adjacent to amalgam (n = 13), resin composites, or other tooth-colored materials (n = 7); three studies assessed both materials. Visual detection included the assessment of discoloration, staining, or other visually detectable changes. Tactile detection focused on the assessment of ditching. Radiographic detection was used both on its own and combined with visual assessment. Laser fluorescence and quantitative light-induced fluorescence were also investigated. Visual (n = 11 studies), radiographic (n = 13), and laser fluorescence detection (n = 8) had similar sensitivities (0.50 to 0.59) and specificities (0.78 to 0.83). Tactile assessment (n = 7) had low accuracy. Light-induced fluorescence (n = 3) was sensitive but showed low specificity. Recent in vitro data found near-infrared light transillumination potentially useful to detect secondary lesions, with similar accuracy as radiographic assessment and being superior to visual-tactile detection [48]. Overall, visual, radiographic, and laser-fluorescence detection are potentially useful to detect secondary caries. Near-infrared transillumination may also be useful, but data remains scarce. For tactile and quantitative light-induced fluorescence assessment, data are both sparse and not promising.
Given that secondary caries detection is performed as part of a routine screening appointment and possibly applied repeatedly, there is some evidence indicating that specific measures should be prioritized, as secondary caries progresses only slowly and missed lesions may be detected at the next screening round. Alternatively, a sequential usage of sensitive followed by specific methods seems advisable [32]. Avoiding false-positive diagnoses, which lead to costly and invasive overtreatment, seems especially relevant in most of today’s low-risk populations.
Dealing with secondary caries: repair or replace restorations?
If secondary caries is detected, dentists are faced with a number of treatment options. Conventionally, such defective restorations were completely removed and replaced. The repeated complete replacement of defective restorations results in excessive removal of dental hard tissue and shortens the lifespan of the tooth [49,50,51]. This is the reason why partial correction, using repair or re-resealing partially defective restorations [52], has become popular [53,54,55]. Such partial corrections are accepted among dentists [53], and a wide range of protocols of how to correct (mainly repair) such restorations exists (see below). Repairs can also be performed for fractured restorations, with fractures being the second main reason for replacement of dental restorations [30].
Repairing restorations is not only considered to preserve tooth structure and reduce the risk of treatment-related complications but also can be less time-consuming and less costly compared with the complete replacement of partially defective restorations. On the other hand, the survival probability of repair restorations might be inferior to that of replaced restorations [56, 57] (Table 1). One aspect assessed by a recent study was if this increase is acceptable given that the original restoration did not need complete removal and replacement in the first place from both an effectiveness and a cost perspective [58]. This study used a modeling methodology to follow repaired or replaced restorations over a lifetime. For partially defective resin composite restorations, the study found repair to be long-term minimally costlier than replacement, but also minimally more effective. For amalgam, repair was much costlier (mainly as complete replacement of amalgam was rather inexpensive) and minimally more effective. The larger (extended) the partially failed restoration, the more cost-effective was the repair, especially for resin composite. Notably, the study assessed if repair was a good option in case the original complication was secondary caries, which is of relevance for the present review. In this case, repair was highly cost-effective (the opposite was the case if fracture was the reason of initial failure). The authors ascribed this to the fact that in restorations failing due to fracture, high physical stress may be the reason for failure and repairs may not be sufficiently suited to withstand this. In contrast, in case of secondary caries, it may not matter too much if the restoration is replaced or repaired as long as the margin integrity is provided. If accessible, repairing or resealing restorations partially defect due to secondary caries seems advisable. More detailed criteria on how to systematically assess the restoration and how to specifically decide between repair versus replacement have been published by a recent review supported by the FDI [59].
The protocols for placement of repair restorations have been discussed in detail elsewhere [60, 61]. Briefly, defective parts of the restoration should be removed, and the restoration surface of the cavity roughened by use of diamond burs. Then, depending on the type of restorative material to repair, different surface pre-treatments can be recommended: Resin composite and amalgam surfaces should be air abraded with Al2O3; silicate ceramic surfaces can also be air abraded with Al2O3. Alternatively, acid etching using hydrofluoric acid can be performed if contamination of gingiva, enamel, and dentin can be avoided. Metallic and oxide ceramic surfaces should be air abraded using either Al2O3.or silica-coated Al2O3 [62]. Areas of dental hard tissue in the cavity to be repaired should be etched with phosphoric acid. Phosphoric acid etchant can also be applied on composite or glass-based ceramic surfaces to obtain a cleaning effect. However, contact of phosphoric acid to metallic surfaces and zirconia should be avoided as this might hamper the adhesion of 10-methacryloyloxydecyl-dihydrogen-phosphate (MDP) containing primers [63]. Then, silane coupling can be recommended; alternatively, universal primers containing components (e.g., silanes, MDP, sulfur-containing monomers) for chemical bonding to a variety of surfaces can be used. Notably, clinical studies found simplified approaches involving only conventional etching and bonding procedure without air abrasion or further surface treatment of the remaining restorations also effective for restoration repair [64].
Overall, repairing partially defective restorations seems to have advantages for the longevity of the initial restoration, may slow down the restorative spiral of ever escalating hard tissue loss, and hence provides longer retention times for teeth. In case secondary caries was the reason for repairs, these may come with a high cost-effectiveness. However, performing repairs is challenging and requires a number of steps, which may be compromised given their high technical requirements. Moreover, only in certain indications (area to be repaired accessible, remaining restoration intact, patients accept repair, repair steps can be performed as described), repairs should be attempted.
Gaps of knowledge
Overall, a wide range of knowledge gaps persist, as outlined above. First, the nature of secondary caries, its pathogenesis, and risk factors have not been fully and deeply been understood. Causes, drivers, and facilitators of secondary caries development need to be captured more deeply, using both in vitro and clinical longitudinal research methodology, to derive diagnostic and therapeutic concepts. Second, detecting secondary caries lesions is dominated by concepts which are derived from primary caries detection, while their validation is lacking. The number of diagnostic accuracy studies is limited, and studies testing any kind of further validity (e.g., prognostic validity) and the impact of secondary caries detection on treatment decisions and the long-term impact emanating from that are even scarcer. Third, for treating secondary caries, most studies assessed different materials being used for full replacement of the failed restoration. Alternative treatment concepts like repair or, more so, reseal as well as further strategies for arresting secondary caries have only been sparsely or not at all investigated.
Conclusions
Secondary caries is defined as a lesion associated with restorations or sealants. The effect of the restorative material itself on the secondary caries seems to be limited. Further factors such as the presence and size of restoration gaps, a patient’s caries risk, or experience of the operator have a more important role. Current detection methods for secondary caries are only sparsely validated and likely prone for the risk of over-detection. Using specific methods may be advisable, especially in low-risk populations. Detected secondary caries can be replaced or, if partially defective, also considered for repair or reseal to increase the longevity of the restoration. Overall, there is sparse data towards the nature of secondary caries and how to control, detect, and treat it.
References
Machiulskiene V, Campus G, Carvalho JC, Dige I, Ekstrand KR, Jablonski-Momeni A, Maltz M, Manton DJ, Martignon S, Martinez-Mier EA, Pitts NB, Schulte AG, Splieth CH, Tenuta LMA, Ferreira Zandona A, Nyvad B (2020) Terminology of dental caries and dental caries management: consensus report of a workshop organized by ORCA and Cariology Research Group of IADR. Caries Res 54(1):7–14
Mjor IA, Toffenetti F (2000) Secondary caries: a literature review with case reports. Quintessence Int 31(3):165–179
Kidd EA (2001) Diagnosis of secondary caries. J Dent Educ 65(10):997–1000
Schwendicke F, Kern M, Blunck U, Dörfer C, Drenck J, Paris S (2014) Marginal integrity and secondary caries of selectively excavated teeth in vitro. J Dent 42(10):1261–1268
Ferracane JL (2017) Models of caries formation around dental composite restorations. J Dent Res 96(4):364–371
Kidd EAM (1990) Caries diagnosis within restored teeth. Adv Dent Res 4(1):10–13
Cenci MS, Pereira-Cenci T, Cury JA, ten Cate J (2009) Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res 43(2):97–102
Diercke K, Lussi A, Kersten T, Seemann R (2009) Isolated development of inner (wall) caries like lesions in a bacterial-based in vitro model. Clin Oral Investig 13(4):439–444
Kuper NK, van de Sande F, Opdam NJ, Bronkhorst EM, de Soet JJ, Cenci MS, Huysmans MC (2015) Restoration materials and secondary caries using an in vitro biofilm model. J Dent Res 94(1):62–68
Maske TT, Kuper NK, Cenci MS, Huysmans MDNJM (2017) Minimal gap size and dentin wall lesion development next to resin composite in a microcosm biofilm model. Caries Res 51(5):475–481
Askar H, Brouwer F, Lehmensiek M, Paris S, Schwendicke F (2017) The association between loading of restorations and secondary caries lesions is moderated by the restoration material elasticity. J Dent 58:74–79
Khvostenko D, Salehi S, Naleway SE, Hilton TJ, Ferracane JL, Mitchell JC, Kruzic JJ (2015) Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent Mater 31(6):702–710
Kuper NK et al (2013) Hydrodynamic flow through loading and in vitro secondary caries development. J Dent Res 92(4):383–387
Vandewalle KS, Ferracane JL, Hilton TJ, Erickson RL, Sakaguchi RL (2004) Effect of energy density on properties and marginal integrity of posterior resin composite restorations. Dent Mater 20(1):96–106
Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR (2011) Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res 90(8):953–968
Tjaderhane L (2015) Dentin bonding: can we make it last? Oper Dent 40(1):4–18
Demarco FF et al (2017) Should my composite restorations last forever? Why are they failing? Braz Oral Res 31(suppl 1):e56
Mjor IA (1985) Frequency of secondary caries at various anatomical locations. Oper Dent 10(3):88–92
Mjor IA (2005) Clinical diagnosis of recurrent caries. J Am Dent Assoc 136(10):1426–1433
Kuper NK et al (2014) Gap size and wall lesion development next to composite. J Dent Res 93(7_suppl):108S–113S
Kuper NK, Opdam NJ, Bronkhorst EM, Huysmans MC (2012) The influence of approximal restoration extension on the development of secondary caries. J Dent 40(3):241–247
Demarco FF, Collares K, Coelho-de-Souza FH, Correa MB, Cenci MS, Moraes RR, Opdam NJ (2015) Anterior composite restorations: a systematic review on long-term survival and reasons for failure. Dent Mater 31(10):1214–1224
Opdam NJ, Bronkhorst EM, Roeters JM, Loomans BA (2007) A retrospective clinical study on longevity of posterior composite and amalgam restorations. Dent Mater 23(1):2–8
van de Sande FH et al (2013) Patient risk factors' influence on survival of posterior composites. J Dent Res 92(7 Suppl):78s–83s
Correa MB, Peres MA, Peres KG, Horta BL, Barros AJ, Demarco FF (2013) Do socioeconomic determinants affect the quality of posterior dental restorations? A multilevel approach. J Dent 41(11):960–967
Laske M, Opdam NJ, Bronkhorst EM, Braspenning JC, Huysmans MC (2016) Longevity of direct restorations in Dutch dental practices. Descriptive study out of a practice based research network. J Dent 46:12–17
Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJ (2012) Longevity of posterior composite restorations: not only a matter of materials. Dent Mater 28(1):87–101
Schwendicke F, Krüger H, Schlattmann P, Paris S (2016) Restoration outcomes after restoring vital teeth with advanced caries lesions: a practice-based retrospective study. Clin Oral Investig 20(7):1675–1681
Brouwer F, Askar H, Paris S, Schwendicke F (2016) Detecting secondary caries lesions: a systematic review and meta-analysis. J Dent Res 95(2):143–151
Mjor IA, Moorhead JE, Dahl JE (2000) Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J 50(6):361–366
Mjor IA et al (2002) Placement and replacement of restorations in general dental practice in Iceland. Oper Dent 27(2):117–123
Schwendicke F, Brouwer F, Paris S, Stolpe M (2016) Detecting proximal secondary caries lesions: a cost-effectiveness analysis. J Dent Res 95(2):152–159
Hetrodt F, Lausch J, Meyer-Lueckel H, Conrads G, Apel C (2019) Evaluation of restorative materials containing preventive additives in a secondary caries model in vitro. Caries Res 53(4):447–456
Kuper NK, Montagner AF, van de Sande F, Bronkhorst EM, Opdam NJ, Huysmans MC (2015) Secondary caries development in in situ gaps next to composite and amalgam. Caries Res 49(5):557–563
Boutsiouki C, Frankenberger R, Lücker S, Krämer N (2019) Inhibition of secondary caries in vitro by addition of chlorhexidine to adhesive components. Dent Mater 35(3):422–433
Hetrodt F et al (2019) Evaluation of restorative materials containing preventive additives in a secondary caries model in vitro. Caries Res:1–10
do Amaral GS et al (2016) Restorative materials containing antimicrobial agents: is there evidence for their antimicrobial and anticaries effects? A systematic review. Aust Dent J 61(1):6–15
Askar H, Tu YK, Paris S, Yeh YC, Schwendicke F (2017) Risk of caries adjacent to different restoration materials: systematic review of in situ studies. J Dent 56:1–10
Hollanders ACC, Kuper NK, Maske TT, Huysmans MDNJM (2018) Secondary caries in situ models: a systematic review. Caries Res 52(6):454–462
Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitão J, DeRouen T (2007) Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc 138(6):775–783
Rasines Alcaraz MG et al (2014) Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth. Cochrane Database Syst Rev 3:Cd005620
United Nations Environmental Programme 2013 Minamata Convention on Mercury. United Nations: New York.
Schwendicke F et al (2018) Amalgam alternatives: cost-effectiveness and value of information analysis. J Dent Res:22034518782671
Österreichische Zahnärztekammer. Einführung folgender neuer Vertragsleistungen. 2018
Laske M et al (2016) Ten-year survival of class II restorations placed by general practitioners. JDR Clinical & Translational Research 3:292–299
Opdam NJ, Bronkhorst EM, Loomans BA, Huysmans MC (2010) 12-year survival of composite vs. amalgam restorations. J Dent Res 89(10):1063–1067
Gordan VV, Riley JL 3rd, Carvalho RM, Snyder J, Sanderson JL, Anderson M, Gilbert GH, DPBRN Collaborative Group (2011) Methods used by Dental Practice-based Research Network (DPBRN) dentists to diagnose dental caries. Oper Dent 36(1):2–11
Elhennawy K, Askar H, Jost-Brinkmann PG, Reda S, al-Abdi A, Paris S, Schwendicke F (2018) In vitro performance of the DIAGNOcam for detecting proximal carious lesions adjacent to composite restorations. J Dent 72:39–43
Gordan VV (2001) Clinical evaluation of replacement of class V resin based composite restorations. J Dent 29(7):485–488
Gordan VV (2000) In vitro evaluation of margins of replaced resin-based composite restorations. J Esthet Dent 12(4):209–215
Gordan VV, Mondragon E, Shen C (2002) Replacement of resin-based composite: evaluation of cavity design, cavity depth, and shade matching. Quintessence Int 33(4):273–278
Green D, Mackenzie L, Banerjee A (2015) Minimally invasive long-term management of direct restorations: the ‘5 Rs’. Dent Update 42(5):413 -6, 419-21, 423-6
Kanzow P et al. 2016 Attitudes, practice and experience of German dentists regarding repair restorations. Clinical Oral Investigations, submitted
Gordan VV, Riley J 3rd, Geraldeli S, Williams OD, Spoto JC 3rd, Gilbert GH, National Dental PBRN Collaborative Group (2014) The decision to repair or replace a defective restoration is affected by who placed the original restoration: findings from the National Dental PBRN. J Dent 42(12):1528–1534
Gordan VV et al (2012) Repair or replacement of defective restorations by dentists in the Dental Practice-Based Research Network. J Am Dent Assoc 143(6):593–601
Opdam NJ et al (2012) Longevity of repaired restorations: a practice based study. J Dent Child (Chic) 40(10):889–835
Smales RJ, Hawthorne WS (2004) Long-term survival of repaired amalgams, recemented crowns and gold castings. Oper Dent 29(3):249–253
Kanzow P, Wiegand A, Schwendicke F (2016) Cost-effectiveness of repairing versus replacing composite or amalgam restorations. J Dent 54:41–47
Hickel R, Peschke A, Tyas M, Mjör I, Bayne S, Peters M, Hiller KA, Randall R, Vanherle G, Heintze SD (2010) FDI World Dental Federation - clinical criteria for the evaluation of direct and indirect restorations. Update and clinical examples. J Adhes Dent 12(4):259–272
Hickel R, Brushaver K, Ilie N (2013) Repair of restorations--criteria for decision making and clinical recommendations. Dent Mater 29(1):28–50
Loomans B, Ozcan M (2016) Intraoral repair of direct and indirect restorations: procedures and guidelines. Oper Dent 41(S7):S68–S78
Onisor I, Bouillaguet S, Krejci I (2007) Influence of different surface treatments on marginal adaptation in enamel and dentin. J Adhes Dent 9(3):297–303
Angkasith P, Burgess JO, Bottino MC, Lawson NC (2016) Cleaning methods for zirconia following salivary contamination. J Prosthodont 25(5):375–379
Opdam NJ et al (2012) Longevity of repaired restorations: a practice based study. J Dent 40(10):829–835
Fernandez E et al (2015) Can repair increase the longevity of composite resins? Results of a 10-year clinical trial. J Dent 43(2):279–286
Martin J, Fernandez E, Estay J, Gordan VV, Mjor IA, Moncada G (2013) Minimal invasive treatment for defective restorations: five-year results using sealants. Oper Dent 38(2):125–133
Gordan VV et al (2011) Alternative treatments to replacement of defective amalgam restorations: results of a seven-year clinical study. 142:842–849
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
HA and FS conceived the study, designed it, performed the searches, interpreted the data, and wrote the manuscript. All other authors interpreted the data, wrote, or revised the manuscript. All authors have read and agreed the final manuscript and agree to be accountable for it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No ethical approval needed.
Informed consent
No informed consent needed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Askar, H., Krois, J., Göstemeyer, G. et al. Secondary caries: what is it, and how it can be controlled, detected, and managed?. Clin Oral Invest 24, 1869–1876 (2020). https://doi.org/10.1007/s00784-020-03268-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03268-7