Abstract
Objectives
To assess the viability of dental pulp stem cells loaded with gold nanoparticles complexed with poly (l-lysine) (AuNP-PLL) and to track the cellular behavior in a 3D analysis by micro-CT.
Materials and methods
DPSC (dental pulp stem cells) were cultured and incorporated with AuNP-PLL (0.2 mg/ml) and assessed for cell viability (24 h, 48 h, and 72 h) using MTS assay. Apoptosis/cell death index and cell cycle were analyzed by propidium iodide. AuNP-PLL-RITC were used for observation in confocal microscopy and quantification of the incorporation rates. Cells were also suspended in agarose and analyzed three-dimensionally in μCT, assessing their radiopacity. Quantitative data (cell viability and apoptosis) were analyzed by t test (p < 0.05).
Results
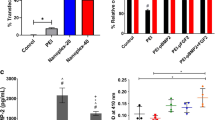
AuNP-PLL labeling did not affect cellular viability in any of the periods analyzed nor interfered with the apoptosis index of DPSC. AuNP-PLL nanocomplexes were identified in the cytoplasm of cells by immunofluorescence, mainly in the perinuclear region. The observed incorporation rate was 98%. Micro-CT analysis has shown that incorporated cells are now visible using x-ray, with a clear increase in radiopacity when compared to the control group (non-incorporated cells).
Conclusion
These results indicate that it is possible to incorporate AuNP-PLL complex into DPSC and track the cells by using μCT; furthermore, this incorporation of 0.2 mg/ml of AuNP-PLL does not interfere in the DPSC basic behavior.
Clinical relevance
This methodology can be a useful tool for cellular labeling to observe cell behavior and their interaction with scaffolds in a 3D manner, opening an array of new approaches in regenerative endodontics.



Similar content being viewed by others
References
Cavalcanti BN, Zeitlin BD, Nör JE (2013) A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater 29:97–102. https://doi.org/10.1016/j.dental.2012.08.002
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962–969. https://doi.org/10.1016/j.joen.2008.04.009
Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, Ariji Y, Matsushita K (2017) Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther 8:61. https://doi.org/10.1186/s13287-017-0506-5
Piva E, Tarlé SA, Nör JE, Zou D, Hatfield E, Guinn T, Eubanks EJ, Kaigler D (2017) Dental pulp tissue regeneration using dental pulp stem cells isolated and expanded in human serum. J Endod 43:568–574. https://doi.org/10.1016/j.joen.2016.11.018
Rosa V, Zhang Z, Grande RH, Nör JE (2013) Dental pulp tissue engineering in full-length human root canals. J Dent Res 92:970–975. https://doi.org/10.1177/0022034513505772
Metscher BD (2009) MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol 9:11. https://doi.org/10.1186/1472-6793-9-11
Kim T, Lee N, Arifin DR, Shats I, Janowski M, Walczak P, Hyeon T, Bulte JWM (2017) In vivo micro-CT imaging of human mesenchymal stem cells labeled with gold-poly-L-lysine nanocomplexes. Adv Funct Mater 27(3). https://doi.org/10.1002/adfm.201604213
Jackson PA, Rahman WN, Wong CJ, Ackerly T, Geso M (2010) Potential dependent superiority of gold nanoparticles in comparison to iodinated contrast agents. Eur J Radiol 75:104–109. https://doi.org/10.1016/j.ejrad.2009.03.057
Zhou C, Long M, Qin Y, Sun X, Zheng J (2011) Luminescent gold nanoparticles with efficient renal clearance. Angew Chem Int Ed Engl 50:3168–3172. https://doi.org/10.1002/anie.201007321
Albukhaty S, Naderi-Manesh H, Tiraihi T, Sakhi Jabir M (2018) Poly-l-lysine-coated superparamagnetic nanoparticles: a novel method for the transfection of pro-BDNF into neural stem cells. Artif Cells Nanomed Biotechnol 46:S125–SS32. https://doi.org/10.1080/21691401.2018.1489272
Pongrac IM, Dobrivojević M, Ahmed LB, Babič M, Šlouf M, Horák D, Gajović S (2016) Improved biocompatibility and efficient labeling of neural stem cells with poly(L-lysine)-coated maghemite nanoparticles. Beilstein J Nanotechnol 7:926–936. https://doi.org/10.3762/bjnano.7.84
Ariji Y, Ariji E, Nakashima M, Iohara K (2018) Magnetic resonance imaging in endodontics: a literature review. Oral Radiol 34:10–16. https://doi.org/10.1007/s11282-017-0301-0
Meschi N, EzEldeen M, Torres Garcia AE, Jacobs R, Lambrechts P (2018) A retrospective case series in regenerative endodontics: trend analysis based on clinical evaluation and 2- and 3-dimensional radiology. J Endod 44:1517–1525. https://doi.org/10.1016/j.joen.2018.06.015
Zhang D, Chen J, Lan G, Li M, An J, Wen X, Liu L, Deng M (2017) The root canal morphology in mandibular first premolars: a comparative evaluation of cone-beam computed tomography and micro-computed tomography. Clin Oral Investig 21:1007–1012. https://doi.org/10.1007/s00784-016-1852-x
Ahrens ET, Bulte JW (2013) Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol 13:755–763. https://doi.org/10.1038/nri3531
Meir R, Shamalov K, Betzer O, Motiei M, Horovitz-Fried M, Yehuda R, Popovtzer A, Popovtzer R, Cohen CJ (2015) Nanomedicine for cancer immunotherapy: tracking cancer-specific T-cells in vivo with gold nanoparticles and CT imaging. ACS Nano 9:6363–6372. https://doi.org/10.1021/acsnano.5b01939
Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F (2009) Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 51:433–445. https://doi.org/10.1016/j.jhep.2009.05.023
Yaghoubi SS, Jensen MC, Satyamurthy N, Budhiraja S, Paik D, Czernin J, Gambhir SS (2009) Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol 6:53–58. https://doi.org/10.1038/ncponc1278
Meir R, Popovtzer R (2018) Cell tracking using gold nanoparticles and computed tomography imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol 10:2. https://doi.org/10.1002/wnan.1480
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1:325–327. https://doi.org/10.1002/smll.200400093
Polak P, Shefi O (2015) Nanometric agents in the service of neuroscience: manipulation of neuronal growth and activity using nanoparticles. Nanomedicine 11:1467–1479. https://doi.org/10.1016/j.nano.2015.03.005
Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH Jr, Bulte JWM (2003) Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology 228:480–487. https://doi.org/10.1148/radiol.2281020638
Astolfo A, Schültke E, Menk RH, Kirch RD, Juurlink BH, Hall C, et al. (2013) In vivo visualization of gold-loaded cells in mice using x-ray computed tomography. Nanomedicine9:284–292. doi:https://doi.org/10.1016/j.nano.2012.06.004
Betzer O, Meir R, Dreifuss T, Shamalov K, Motiei M, Shwartz A, Baranes K, Cohen CJ, Shraga-Heled N, Ofir R, Yadid G, Popovtzer R (2015) In-vitro optimization of nanoparticle-cell labeling protocols for in-vivo cell tracking applications. Sci Rep 5:15400. https://doi.org/10.1038/srep15400
Chhour P, Naha PC, O'Neill SM, Litt HI, Reilly MP, Ferrari VA, Cormode DP (2016) Labeling monocytes with gold nanoparticles to track their recruitment in atherosclerosis with computed tomography. Biomaterials 87:93–103. https://doi.org/10.1016/j.biomaterials.2016.02.009
Meir R, Betzer O, Motiei M, Kronfeld N, Brodie C, Popovtzer R (2017) Design principles for noninvasive, longitudinal and quantitative cell tracking with nanoparticle-based CT imaging. Nanomedicine 13:421–429. https://doi.org/10.1016/j.nano.2016.09.013
Betzer O, Shwartz A, Motiei M, Kazimirsky G, Gispan I, Damti E, Brodie C, Yadid G, Popovtzer R (2014) Nanoparticle-based CT imaging technique for longitudinal and quantitative stem cell tracking within the brain: application in neuropsychiatric disorders. ACS Nano 8:9274–9285. https://doi.org/10.1021/nn503131h
Acknowledgements
The authors would like to acknowledge use of the University of Iowa Central Microscopy Research Facility, a core resource supported by the Vice President for Research & Economic Development, the Holden Comprehensive Cancer Center and the Carver College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, ethical approval is not required.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biz, M.T., Cucco, C. & Cavalcanti, B.N. Incorporation of AuNP-PLL nanocomplexes in DPSC: a new tool for 3D analysis in pulp regeneration. Clin Oral Invest 24, 1761–1767 (2020). https://doi.org/10.1007/s00784-019-03037-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03037-1