Abstract
Objectives
This study investigated and compared the effectiveness of a phytotherapeutic drug composed of herbal extracts on postsurgical discomfort after mandibular third molar surgery.
Materials and methods
Eighty-two patients requiring the surgical removal of a mandibular third molar were randomly assigned to receive placebo (group 1), ibuprofen (group 2), and a phytotherapeutic drug (composed of baicalin, 190 mg; bromelain, 50 mg; escin, 30 mg) (group 3). Drugs were administered after tooth extraction twice a day for 5 days. The primary outcome, pain, was evaluated using a visual analogue scale at 2 h, 6 h, 12 h, 24 h, 48 h, and 7 and 10 days after surgery. The secondary outcomes were the changes in maximum mouth opening and facial contours (mm) between baseline and at 24 h, 72 h, and 7 and 10 days after surgery.
Results
Compared to the baseline, all treatments demonstrated an improvement in the primary and secondary outcomes. Moreover, compared to groups 1 and 2, patients in group 3 yielded a significant reduction of the postoperative pain score at 12 h (p < 0.001), 24 h (p = 0.010), and 48 h (p = 0.048) after surgery. The mean reduction of the swelling and trismus was similar between groups.
Conclusions
The results of this study suggest that a postoperative administration of a phytotherapeutic drug was found to be effective in postoperative pain management after the surgical removal of impacted mandibular third molars.
Clinical relevance
The phytotherapeutic drug composed of herbal extract determined a decrease in the severity of postoperative pain compared to ibuprofen and placebo.
Similar content being viewed by others
Introduction
Extraction of impacted third molars is one the most common surgical procedures in oral surgery [1]. The most commonly observed postoperative complications associated with the removal of mandibular third molars are pain, trismus, and swelling as a result of the local inflammatory process [2]. After every surgical intervention, inflammatory mediators such as prostaglandins, leukotrienes, and platelet-activating factors are released in response to the surgical injury, with a subsequent increase in vascular dilatation and permeability, causing edema and enhancing interstitial tissue response [3].
To manage postoperative discomfort, many strategies have been developed for minimizing clinical manifestations after surgery through a pharmacological approach by inhibiting the synthesis and/or release of the inflammatory mediators of acute inflammation. Among these, corticosteroids and nonsteroidal anti-inflammatory drug (NSAID) have shown immunosuppressive, anti-inflammatory, and analgesic effects [4, 5]. These anti-inflammatory effects are caused in large part by a subsequent decrease in serotonin turnover in the central nervous system, which leads to central inhibition of prostaglandin E2 (PGE2) synthesis that influences the entire systemic inflammatory response [6].
However, the use of corticosteroids or NSAIDs has been associated with some adverse effects such as gastrointestinal bleeding, renal function disturbance, a reduction in platelet function, shortness of breath, and profound hypotension [7]. Moreover, it has been shown that if prolonged over time, these therapies can result in a strong reduction in immune defenses [8, 9].
In light of these limitations, complementary protocols have been suggested for the postsurgical therapy of third molar surgery.
For many years, many phytotherapeutic drugs have been widely used for the treatment of inflammatory diseases and postsurgical conditions [10, 11]. Of these, bromelain has been shown to possess anti-inflammatory activity [12] through the reduction of pain mediators such as prostaglandin E2 and substance P [13], and edema-prophylactic/reducing properties [14, 15]. Among the major anti-inflammatory activities exerted by bromelain, it is presumed that the anti-edema action is such that provides the drug with the greatest therapeutic benefit thanks to the presence of proteases present in its composition that are active during the post-traumatic and postoperative convalescence period following third molar surgery [16, 17]. Moreover, the oral administration of bromelain during the perioperative period for third molar extractions has been showed to effectively improve the quality of life [18, 19].
Baicalin, a natural molecule found in the roots of Baical skullcap (Scutellaria baicalensis Georgi), has been widely used for the clinical treatment of common inflammatory diseases [20]. Baicalin has been shown to possess a variety of interesting properties such as antibacterial [20], antiviral [21], antitumor [22], and anti-inflammatory effects [23]. Moreover, a recent study has shown that baicalin can enhance osteoblast bone formation activities via the Wnt/B-catenin signaling pathway [24] and can downregulate several inflammation-associated genes such as inducible nitric oxide synthase, cyclooxygenases, and lipoxygenases and the relative inhibition of the production prostaglandin E [25].
Similarly to baicalin, escin is a natural molecule with a major active component of Aesculus hippocastanum. Escin is a natural mixture of triterpene saponins and has shown clinically significant anti-inflammatory activity postoperatively and in traumatic injuries [26]. It mainly exerts its anti-inflammatory and anti-edematous effects through its anti-histamine and anti-serotoninergic activities, and by reducing the adhesiveness and migration of neutrophils. It has been previously shown in an in vitro model of induced periodontitis, that escin attenuates, in a concentration-dependent manner, the increase of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) [27].
Subsequently, a new generation of phytotherapeutic drugs with a mixture of bromelain, baicalin, and escin was synthesized to replace those previously used, with encouraging results of a prolonged anti-inflammatory activity [28]. In light of these findings, the aim of the present study was to evaluate the effect of a new generation of a phytotherapeutic drug versus a commonly used anti-inflammatory drug and a placebo on the postoperative discomfort management after third molar surgery. The null hypothesis to invalidate was that, after the last follow-up, there were no variations between the phytotherapeutic drug and the other two groups.
Materials and methods
Ethical issues and trial design
The study was designed as a single-center, randomized, triple-blind, controlled clinical trial. This study followed the Declaration of Helsinki on medical protocol, and the institutional review board of the University of Messina approved the study protocol (no. 9/17). The study was registered at clinicaltrials.gov (ID: NCT03335683). Each patient was informed about the possible risks of the study and provided his written informed consent before any study procedures were performed.
The patient population was randomly and consecutively recruited from the ASA I (American Society of Anesthesiology classification, normal healthy) patients, aged ≥ 18 years requiring surgical removal of a mandibular impacted third molar. Patient recruitment was conducted between January 2016 and September 2017 at the School of Dentistry of the University of Messina, Messina, Italy.
The inclusion criteria were as follows: (1) age between 18 and 32 years; (2) good general health; (3) the presence of at least one asymptomatic mandibular third molar with class II position, type B impaction [29]; (4) absence of pericoronitis or signs of inflammation during the last 30 days. Orthopantomography (OPT) was used to determine tooth position. The exclusion criteria were (1) any systemic condition which might compromise the outcome of surgery or contraindicate the administration of the research drugs; (2) taking medication; (3) use of hormonal contraceptives; (4) status of pregnancy or lactation; (5) previous history of excessive drinking; (6) allergy to local anesthetic; and (7) smoking.
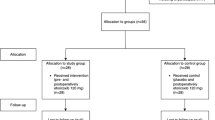
The study was performed according to the CONSORT (Consolidated Standards Of Reporting Trials) guidelines (Fig. 1, Appendix 1) [30]. Patients who did not attend the second surgery or were unable to follow the study protocol were excluded, as were those whose surgical time exceeded 40 min.
Study sample and sample size analysis
During the first phase of the study, 104 patients (55 men, 49 women) were initially enrolled from those referred to the School of Dentistry of the University of Messina. However, after screening, 20 patients (12 men, 8 women) were excluded because they did not meet the exclusion criteria (n = 9), they declined to participate (n = 6), or they were absent during the first visit (n = 5). Thus, the final number of patients assessed for eligibility was 84 (Fig. 1).
All patients underwent an initial preoperative screening consultation by the same experienced clinician, blinded as to the administration of test medication. Patient data were recorded preoperatively and included gender, age, systemic conditions, periodontal status, hemogram parameters, platelet count, international normalized ratio (INR) value [31], and glycated hemoglobin levels. Following inclusion criteria, OPT was examined in order to re-evaluate tooth variables such as position, tooth/root formation, and degree of impaction of the third molar.
The sample size was established by considering an effect size of 0.40 with α = 0.050 and a power level of 0.80 for the variable pain that was the primary outcome variable chosen. The variable “pain” showed a mean difference between groups of 0.55 with a standard deviation (SD) of 1.25. On the basis of these values, a minimum sample size of 69 patients was calculated to be necessary. Considering that some patients could be lost during the follow-up sessions, 84 patients were finally included, so that the primary variable achieved a power value of 0.89.
In the inter-examiner reliability test, the percent agreement was 84.3% (kappa = 0.60) for pain value. The intra-examiner agreement was calculated by Cohen’s k coefficient, which was 0.816, that predicted a good degree of reliability. The kappa coefficients were calculated for the measurements obtained at each different examination. Good reliability (ICC = 0.775) was found for all examinations.
Subjects were allocated to one of three groups according to the medication received: group 1, placebo (sugar pill, Sucratol - Placebo Capsules), twice a day for 5 days; group 2, ibuprofen 600 mg, twice a day for 5 days; group 3, a phytotherapeutic drug (Lenidase®, Enfarma SRL, Misterbianco, Italy), twice a day for 5 days. The phytotherapeutic drug used in the present study (Lenidase®) contained a mixture of herbal extracts such as baicalin (190 mg), extracted from Scutellaria baicalensis; bromelain (50 mg), extract from Ananas comosus; and escin (30 mg), extract from Aesculus hyppocastanum.
All patients in the study routinely received a prophylactic preoperative dose of oral antibiotic (1 g amoxicillin/clavulanic acid 1 h before surgery) (Augmentin; GlaxoSmithKline, Verona, Italy) and a postoperative gastric protectant (20 mg pantoprazole; Sanofi Aventis, Brindisi, Italy).
All patients extended the therapy up to 10 days if they felt discomfort corresponding to a score of 3 on the visual analogue scale (VAS) score for pain [32]. Chlorhexidine-based mouthwash (0.12%) was also prescribed, for use three times a day for 7 days, starting 24 h after surgery. After surgery, the use of antibiotics was not prescribed.
Randomization
Information on the type of medication prescribed to each subject enrolled in the study was blinded to the patient, surgeon, statistician, and clinical investigator responsible for patient follow-up and outcome examinations. A clinician, not involved in the trial, generated a random group allocation sequence by a ratio of 1:1 using a permuted block design by a computer random-number generator. In every patient, one lower impacted third molar was allocated to receive one of the postoperative treatments. The allocation concealment to the therapist was performed through serially numbered sealed envelopes, and the details of the sequence were unidentified to the clinicians participating in the study. Before every treatment, an investigator not involved in the recording and processing of data performed the assignment of the sealed envelopes marked with the initials of the patient’s name and date of birth and containing treatment methods for the therapist for each selected tooth. The groups were coded with the letter “A” representing group 1, letter “B” representing group 2, and letter “C” representing group 3. The envelope decoding this information was only accessed once both the clinical trial and statistical analysis had been concluded. At the same time, each patient and surgical site assigned to receive the postoperative drug therapy was identified. Shortly after each treatment session, another clinician opened the envelope with the assigned number by which the postoperative therapy would subsequently be identified. The same operator performed all the procedures and was blinded to previously recorded data, thus avoiding bias in the evaluation of the experimental data.
Surgery
To minimize differences due to operator variability, all of the surgical extractions were performed by the same oral surgeon. Each patient had similar operative procedures, in the same operating room and under similar conditions, using a uniform local anesthetic technique which included inferior alveolar, lingual, and long buccal nerve blocks using mepivacaine 2% with epinephrine 1:100.000 (2% Carbocaine; AstraZeneca, Milan, Italy) without sedative premedication. The same surgical procedure was adopted for all groups, aiming to reduce the bias related to the intraoperative trauma. Access to the third molar was achieved from the buccal aspect. A mucoperiosteal flap was raised, and bone removal with a round bur in a straight handpiece under continuous irrigation with a sterile saline solution and/or tooth sectioning was performed. After tooth extraction, the alveolus was inspected, curetted for granulation tissue removal, and irrigated with a sterile saline solution. The surgical wound was closed using a 4–0 reabsorbable suture (Coated VICRYL® polyglactin 910, Ethicon, USA). Immediately at the end of surgery, each enrolled patient received the first allocated postoperative tablet.
Postoperative instructions were read and explained carefully to the patient, e.g., following a liquid and cold diet for 24 h, performing rigorous oral hygiene, and avoiding mouthwashes to prevent the occurrence of postsurgical bleeding. Patients were informed that they must contact the surgeon if they experienced persistent bleeding or any other complications such as fever. In addition, patients were also asked to report any physical symptoms experienced during the study period, e.g., nausea, vomiting, headache, and signs of infection. Any enrolled patient who required a single-dose rescue analgesic medication (ketoprofen 80 mg, Dompè, L’Aquila, Italy) was asked to record the number and the frequency of drug intake.
Outcome measures
Immediately after the operation, details of each procedure were recorded, together with the duration of surgery in minutes. The primary outcome variable measured was the occurrence of postoperative pain. This allowed the patient to describe their discomfort more objectively. Postoperative pain intensity was measured using a 10-cm visual analogue scale (VAS), which consisted of an interval scale ranging from 0 (absence of pain or discomfort) to 10 (maximum pain or discomfort). Patients were instructed to record their pain score at 30 min after surgery and then at 2, 6, 12, 24, and 48 h and 7 and 10 days following surgery. Additional analyses included the length of time which elapsed until the intake of a rescue analgesic by the patient. The number of patients requiring a rescue analgesic was also recorded [32].
The occurrence of postoperative inflammatory events was the secondary outcome measure adopted. The differences between preoperative values (baseline) and those measured at 24 and 72 h and 7 and 10 days after surgery were compared. The following measurements were performed to evaluate postoperative swelling on the facial side receiving surgery (Fig. 2): tragus to the nasal border (Tr–Al); tragus to the soft pogonion (Tr–Pog′), tragus to the external corner of the eye (Tr–Exo), tragus to the labial commissure (Tr–Che), angle of the mandible to the soft pogonion (Go–Pog′), angle of the mandible to the external corner of the eye (Go–Exo), angle of the mandible to the nasal border (Go–Al), and angle of the mandible to the labial commissure (Go–Che). To estimate trismus, maximum mouth opening was measured in millimeters between the upper and lower central incisors using a calibrated sliding caliper (Therabite Range of Motion Scales), preoperatively (baseline) and at 24 and 72 h and 7 days after surgery. The reduction in mouth opening was calculated as the difference between the preoperative value (baseline) and the postoperative value at each time point.
Facial measurements for the assessment of postoperative swelling in the study groups. Blue lines: tragus to the external corner of the eye (Tr–Exo), tragus to the nasal border (Tr–Al), tragus to the labial commissure (Tr–Che), and tragus to the soft pogonion (Tr–Pog′). Red lines: angle of the mandible to the external corner of the eye (Go–Exo), angle of the mandible to the nasal border (Go–Al), angle of the mandible to the soft pogonion (Go–Pog′), and angle of the mandible to the labial commissure (Go–Che)
Statistical analysis
The examined data was normally distributed and verified by the Kolmogorov–Smirnov test; consequently, we applied a parametric approach for data analysis. The Mann–Whitney test was used for comparisons of pain scores and facial distances between groups. The Friedman test (Dunn post hoc test) was used to assess the same variables (pain score and facial distances) among each follow-up session. One-way analysis of variance (ANOVA; Tukey post hoc tests) and the Students t test for paired samples were used to assess the means of maximum mouth opening for each follow-up session. The significance of the p value was set at 0.05. All statistical analyses were executed using a software program (SPSS 17.0 for Window package, IBM, Chicago, IL, USA).
Results
All enrolled patients completed the study without any postoperative complications. The mean age of the 84 patients assessed for eligibility (45 males, 39 females) was 26.8 years (± 4.2). Two patients were excluded from analysis because he used a rescue drug not adopted in the study. The final number of the analyzed patients was 82 (Fig. 1). The postoperative course was unremarkable in all patients during the follow-up without any adverse events such as abscesses or infections.
The total amount of local anesthetic injected at each surgical site was measured based on the total number of dental cartridges used per procedure. There was no significant difference in the mean number of dental cartridges used between the groups (group 1, 2.4 ± 0.3; group 2, 2.2 ± 0.2; group 3, 2.1 ± 1.6; p = 0.278). The mean duration of surgery was 24.28 ± 7.29 min for group 1, 23.36 ± 6.25 min for group 2, and 26.29 ± 6.27 min for group 3, not statistically significant (p = 0.188). All patients underwent osteotomy and tooth sectioning without intraoperative accidents or complications.
Pain levels
One case in group 1 required a single-dose rescue analgesic medication (ketoprofen 80 mg, Dompè, L’Aquila, Italy) at 12 h after surgery, while none of the patients in groups 2 and 3 required adjunct analgesic medication. The postoperative peak pain score occurred at 12 h in group 1, 10 h in group 2, and 6 h in group 3 (Fig. 3). A further comparison between groups highlighted that the mean pain score values (VAS) were significantly lower only in group 3 at 12 h (p < 0.001), 24 h (p = 0.010), and 48 h (p = 0.048) after surgery compared to groups 1 and 2, while the other time points were not significantly different (Fig. 3, Table 1).
The Dunn post hoc test identified a statistically significant difference found between the time points in group 1 between 12 h and 7 days and between 72 h and 7 days (Fig. 4). In group 2, a difference between the time points was found between 6 h, 72 h, and 7 days. In group 3, a difference between the time points was found between 6 h, 12 h, 72 h, and 7 days (Fig. 4).
Swelling and trismus
Postoperative facial measurements in the mean linear distances, even if lower in group 3, did not differ between the groups at each observation point (Fig. 2, Table 2). The values of maximum mouth opening did not differ between groups (p > 0.05) (Table 3). However, a further comparison between groups showed a statistically significant difference in the maximum mouth opening when the baseline values were compared to the postoperative ones (group 1, 72 h and 7 days, p < 0.05; group 2, 24 h, 72 h, and 7 days, p < 0.05; group 3, 24 h, 72 h, and 7 days, p < 0.05) (Fig. 5). Moreover, compared to other groups, there was a statistically significant difference in maximum mouth opening at 7 days postoperative (p < 0.05) in group 3 when compared with the 24-h postoperative period (Fig. 5).
Discussion
This randomized controlled clinical trial was aimed at comparing the effect of an administration of a phytotherapeutic drug with anti-inflammatory activity in the prevention of postoperative discomfort after third molar surgery compared to ibuprofen and to placebo. The specific aims of the study were to evaluate the effectiveness of a phytotherapeutic drug on postoperative pain, facial swelling, and mouth opening in the mandibular third molar surgery.
The treatment with a phytotherapeutic drug determined a statistically relevant decrease in the severity of postoperative pain compared to ibuprofen and placebo over time.
The third molar surgery model was chosen because it is a widespread procedure in which postoperative pain is usually observed in the early stages after the procedure [33].
One of the key points of the third molar surgery is to reduce severe postoperative discomfort, a critical step that has been shown to be necessary to the success of this kind of procedure [34]. Thus, many clinicians have attempted to reduce postsurgical sequelae by using anti-inflammatory drugs. The anti-inflammatory efficacy of corticosteroids and NSAIDs has been widely proved in the surgical removal of third molars [35]. However, several pharmacological studies have demonstrated a poor tolerance or adverse effects in patients that underwent NSAIDs or corticosteroids following their administration. Olmedo et al. [36] in their study showed that 37.3% of the patients who required adjuvant treatment with ketorolac and ibuprofen following third molar surgery reported some type of adverse effect, with drowsiness being the most prevalent (10.7% of cases), followed by gastric disturbance (8%) and dizziness (5.3%). The most prevalent adverse effect related to ketoprofen was pyrosis (10.3%). Three serious adverse effects were reported by Olson et al. [37], with two of these events related to ibuprofen and one related to acetaminophen. Moreover, many strong NSAIDs have been associated with a risk of gastric irritation [38]. Thus, greater efforts are being made to find new treatment strategies that may not rely on adverse effects of NSAIDs or corticosteroids [34].
Among NSAIDs, several new selective COX-2 inhibitors have recently been demonstrated, in different study models, to be efficacious for pain management following surgical extraction of impacted teeth [39, 40]. Yamashita et al. [41] found that when Celecoxib was used to manage postoperative discomfort following third molar surgery, its efficacy was comparable to loxoprofen in the early stages for acute pain management. Moreover, the positive effects of celecoxib were also associated with fewer episodes of pyrosis and upper and lower gastrointestinal lesions compared to other NSAIDs [42].
However, for several decades, third molar surgery has frequently been enhanced by the use of natural substances that could offer many potential benefits in helping to treat inflammatory conditions, especially pain [43]. The anti-inflammatory and analgesic properties of bromelain have been already demonstrated in vitro and through human studies in patients who have experienced major trauma [15]. Besides immunomodulating and anti-inflammatory properties, an important indication for bromelain therapy is postoperative and post-traumatic swelling without any gastric effects. In our study, no adverse effect, such as gastrointestinal discomfort, dizziness, or nausea, was reported by the use of the phytotherapeutic drug.
Our research has focused on a novel natural product, which is a concentrated mixture of herb extracts that possess an anti-inflammatory and anti-oedemigenous activity [44]. This agent contains a mixture of 50 mg of bromelin; 190 mg of baicalin, a flavonoid from Scutellaria baicalensis that inhibits 5-lipoxygenase (5-LOX) and COX activities and leukotriene synthesis [45]; and 30 mg of escin, extract from Aesculus hyppocastanum, which has an anti-edemigenous effect that enhances endogenous corticosteroid receptor activity [45, 46].
de la Barrera-Núñez et al. [19] had reported that, when bromelin alone was used as postoperative therapy following third molar surgery, no significant difference was found in inflammation, pain, and mouth opening. Moreover, a further study on third molar surgery by Bornmann et al. [47] showed that the use of bromelain was effective for the reduction of postoperative edema and inflammatory reactions.
Based on the pilot observation by Bornmann et al. [47], we designed the current study to compare the clinical and anti-inflammatory effects of novel phytotherapeutic drugs containing herbal extract mixture of bromelain, baicalin, and escin in the treatment of edematous swelling, localized edema, and pain following extraction of mandibular impacted third molars.
In the present study, the phythoterapeutic drug tested showed a significant analgesic effect during the first postoperative week compared with ibuprofen and with placebo. Considering the time intervals assessed in the present study to evaluate postoperative pain (2, 6, 12, 24, and 48 h and 7 and 10 days), the peak in pain occurred only at 6 h after the surgical procedure when the phytotherapeutic drug was used; then, the pain values decreased in the other follow-up sessions. Differently, in the other group, the peak in pain occurred at 12 h and 24 h and remained higher up to 72 h after surgery. Moreover, the phythoterapeutic drug group showed a mean lower pain score at 6-, 12-, 24-, and 48-h intervals compared to the other groups. These results demonstrated a better analgesic efficacy of the phythoterapeutic drug in the first phase of healing.
Our findings are consistent with those reported in recently published reports showing that bromelain provides significant postoperative short-term clinical benefits following third molar surgery [18, 43]. It is believed that such anti-edema effects are due to all of the active proteases of bromelain that act positively during the early stages after surgery [16]. On the other hand, the effects of baicalin were reported to be due to its anti-inflammatory and analgesic mechanisms associated with the inhibition of critical inflammatory mediators, including nitric oxide, PGE 2, and pro-inflammatory cytokines, accompanied by an increase in IL-10 production (that play a critical role in stimulating the innate host response) at sites of inflammation [48, 49]. Both of the effects exerted by bromelain and baicalin could be enhanced by escin. In fact, it was shown that escin presented similar and longer anti-inflammatory effects compared to dexamethasone. Its main activity was observed in the enhancement of blood capillary permeability and to stimulate lymphatic drainage, without any immunosuppressive effects [50].
With regard to swelling values, there was no significant difference between groups, even if they were lower in the phytotherapeutic drug group. In both groups, p values were less than 0.05 for the differences in measurements between the preoperative and the postoperative periods for facial contour measurements, in accordance with other studies in which dexamethasone and nimesulide [51] or ibuprofen [52] were used.
However, regarding swelling, the results of the present study were different when compared with other studies in which the phytotherapeutic drugs were used [47]. This could be justified because swelling evaluation in the present study was recorded with clinical measurements of the facial contours, not so reliable as well as other tools such as 3D face scanning systems.
Indeed, a number of different techniques have been previously used to measure postoperative swelling following third molar surgery, including verbal response scales, mechanical methods (cephalostats, calipers, and registration of reference points or landmarks), ultrasound, photographic techniques, computed tomography, and magnetic resonance imaging [53, 54]. Advances in 3D imaging techniques allowed to capture and superimpose facial images and measure alterations in soft tissue position in three dimensions [55].
Based on these findings, a future study with a swelling evaluation by soft tissue images obtained with some 3D systems could provide a more reliable evaluation of the facial contours with better accuracy and reproducibility.
The assessment of trismus, performed by measuring the difference in maximum mouth opening between the postoperative and preoperative periods, showed a decrease observed in the first 24 and 72 h after surgery in the phytotherapeutic drug group [56,57,58]. These results are in accordance with the study performed by De Menezes and Cury [59], in which patients, however, used nimesulide, as well as in the study of Bjornsson et al. [60], in which however ketoprofen was tested.
During recent years, a number of drugs have been studied for the reduction in postoperative discomfort after third molar surgery. There is ongoing research to develop and examine new agents that can help in the reduction of pain and swelling following surgery [61, 62] without causing adverse effects. Identifying new agents should be encouraged in order to find alternative treatment strategies to NSAIDs or corticosteroids.
This study indicated that, when the phytotherapeutic drugs were used as a postoperative therapy of third molar surgery, there were favorable effects in the inflammatory parameters. The phytotherapeutic drug as a mixture of herbal extract with anti-inflammatory activity was demonstrated to be safe and simple in the control of pain control and postsurgical discomfort after surgical removal of the third molar.
This initial study is promising and demands further studies to understand better the role and potential benefits of phytotherapeutic drugs in the postoperative therapy of impacted third molar surgery.
References
Brković B, Andrić M, Ćalasan D, Milić M, Stepić J, Vučetić M, Brajković D, Todorović L (2017) Efficacy and safety of 1% ropivacaine for postoperative analgesia after lower third molar surgery: a prospective, randomized, double-blinded clinical study. Clin Oral Investig 21:779–785
Bataineh AB, Batarseh RA (2017) The effect of modified surgical flap design for removal of lower third molars on lingual nerve injury. Clin Oral Investig 21:2091–2099
Bamgbose BO, Akinwande JA, Adeyemo WL, Ladeinde AL, Arotiba GT, Ogunlewe MO (2005) Effects of co-administered dexamethasone and diclofenac potassium on pain, swelling and trismus following third molar surgery. Head Face Med 1:11
Isola G, Matarese G, Cordasco G, Perillo L, Ramaglia L (2016) Mechanobiology of the tooth movement during the orthodontic treatment: a literature review. Minerva Stomatol 65:299–327
Kaplan V, Eroğlu CN (2016) Comparison of the effects of daily single-dose use of flurbiprofen, diclofenac sodium, and tenoxicam on postoperative pain, swelling, and trismus: a randomized double-blind study. J Oral Maxillofac Surg 74:1946.e1–1946.e6
Ferrari AM, Byers MR (1996) Chronic dexamethasone treatment and its effects on sensory neuropeptides, pulpal injury reactions and reparative dentin. Brain Res 723:125–134
Piecuch JF (2012) What strategies are helpful in the operative management of third molars? J Oral Maxillofac Surg 70(9 Suppl 1):S25–S32
Ghaeminia H, Hoppenreijs TJ, Xi T, Fennis JP, Maal TJ, Bergé SJ, Meijer GJ (2017) Postoperative socket irrigation with drinking tap water reduces the risk of inflammatory complications following surgical removal of third molars: a multicenter randomized trial. Clin Oral Investig 21:71–83
Isola G, Matarese G, Williams RC, Iorio-Siciliano V, Alibrandi A, Cordasco G, Ramaglia L (2018) The effects of a desiccant agent in the treatment of chronic periodontitis: a randomized, controlled clinical trial. Clin Oral Investig 22:791–800
Pavan R, Jain S, Shraddja KA (2012) Properties and therapeutic application of bromelain: a review. Biotechnol Res Int 2012:976203
Olmedo-Gaya MV, Manzano-Moreno FJ, Galvez-Mateos R, González-Rodriguez MP, Talero-Sevilla C, Vallecillo-Capilla M (2016) Oral pregabalin for postoperative pain relief after third molar extraction: a randomized controlled clinical trial. Clin Oral Investig 20:1819–1826
Müller S, März R, Schmolz M, Drewelow B, Eschmann K, Meiser P (2012) Placebo-controlled randomised clinical trial on the immunomodulating activities of low and high dose bromelain after oral administration – new evidence on the anti-inflammatory mode of action of bromelain. Phytother Res 27:199–204
Gaspani L, Limiroli E, Ferrario P, Bianchi M (2002) In vivo and in vitro effects of bromelain on PGE2 and SP concentrations in the inflammatory exudate in rats. Pharmacology 65:83–86
de-Azevedo-Vaz SL, Oenning AC, Felizardo MG, Haiter-Neto F, de Freitas DQ (2015) Accuracy of the vertical tube shift method in identifying the relationship between the third molars and the mandibular canal. Clin Oral Investig 19:583–588
Maurer HR (2001) Bromelain: biochemistry, pharmacology and medical use. Cell Mol Life Sci 58:1234–1245
Enomoto T, Mineshita S, Shigei T (1968) Protective effect of stem bromelain against adrenaline pulmonary edema and its dependence on the proteolytic activity. Jpn J Pharmacol 18:260–265
Singh T, More V, Fatima U, Karpe T, Aleem MA, Prameela J (1968) Effect of proteolytic enzyme bromelain on pain and swelling after removal of third molars. J Int Soc Prev Community Dent 6:S197–S204
Majid OW, Al-Mashhadani BA (2014) Perioperative bromelain reduces pain and swelling and improves quality of life measures after mandibular third molar surgery: a randomized, double-blind, placebo-controlled clinical trial. J Oral Maxillofac Surg 72:1043–1048
de la Barrera-Núñez MC, Yáñez-Vico RM, Batista-Cruzado A, Heurtebise-Saavedra JM, Castillo-de Oyagüe R, Torres-Lagares D (2014) Prospective double-blind clinical trial evaluating the effectiveness of bromelain in the third molar extraction postoperative period. Med Oral Patol Oral Cir Bucal 19:e157–e162
Li HY, Hu J, Zhao S, Yuan ZY, Wan HJ, Lei F, Ding Y, Xing DM, Du LJ (2012) Comparative study of the effect of baicalin and its natural analogs on neurons with oxygen and glucose deprivation involving innate immune reaction of TLR2/TNF alpha. J Biomed Biotechnol 2012:267890
Moghaddam E, Teoh BT, Sam SS, Lani R, Hassandarvish P, Chik Z, Yueh A, Abubakar S, Zandi K (2014) Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci Rep 4:5452
Chen JC, Li ZL, Chen AY, Ye X, Luo H, Rankin GO, Chen YC (2013) Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int J Mol Sci 14:6012–6025
Lee W, Ku SK, Bae JS (2015) Anti-inflammatory effects of baicalin, baicalein, and wogonin in vitro and in vivo. Inflammation 38:110–125
Chi YS, Lim H, Park H, Kim HP (2003) Effects of Wogonin, a plant flavone from Scutellaria radix, on skin inflammation: in vivo regulation of inflammation associated gene expression. Biochem Pharmacol 66:1271–1278
Ma SC, Du J, But PP, Deng XL, Zhang YW, Ooi VE, Xu HX, Lee SH, Lee SF (2002) Anti-viral Chinese medicinal herbs against respiratory syncytial virus. J Ethnopharmacol 79:205–211
Wang T, Fu FH, Zhang LM, Han B, Zhu M, Zhang X (2009) Effect of of escin on acute inflammation and the immune system in mice. Pharmacol Rep 61:697–704
Liu S, Wang H, Qiu C, Zhang J, Zhang T, Zhou W, Lu Z, Rausch-Fan X, Liu Z (2012) Escin inhibits lipopolysaccharide-induced inflammation in human periodontal ligament cells. Mol Med Rep 6:1150–1154
Candiano G, Pepe P, Pietropaolo F, Aragona F (2013) Does prolonged anti-inflammatory therapy reduce number of unnecessary repeat saturation prostate biopsy? Arch Ital Urol Androl 85:65–68
Pell GJ, Gregory BT (1933) Impacted mandibular third molars: classification and modified techniques for removal. Dent Dig 39:330–338
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ et al (2012) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 10:28–55
Isola G, Matarese G, Cordasco G, Rotondo F, Crupi A, Ramaglia L (2015) Anticoagulant therapy in patients undergoing dental interventions: a critical review of the literature and current perspectives. Minerva Stomatol 64:21–46
Ong KS, Lirk P, Tan JML, Sow BW (2005) The analgesic efficacy of intravenous versus oral tramadol for preventing postoperative pain after third molar surgery. J Oral Maxillofac Surg 63:1162–1168
Wang D, He X, Wang Y, Li Z, Zhu Y, Sun C, Ye J, Jiang H, Cheng J (2017) External root resorption of the second molar associated with mesially and horizontally impacted mandibular third molar: evidence from cone beam computed tomography. Clin Oral Investig 21:1335–1342
Au AH, Choi SW, Cheung CW, Leung YY (2015) The efficacy and clinical safety of various analgesic combinations for post-operative pain after third molar surgery: a systematic review and meta-analysis. PLoS One 10:e0127611
Gersema L, Baker K (1992) Use of corticosteroids in oral surgery. J Oral Maxillofac Surg 50:270–277
Olmedo MV, Galvez R, Vallecillo M (2001) Double-blind parallel comparison of multiple doses of ketorolac, ketoprofen and placebo administered orally to patients with postoperative dental pain. Pain 90:135–141
Olson NZ, Otero AM, Marrero I, Tirado S, Cooper S, Doyle G, Jayawardena S, Sunshine A (2001) Onset of analgesia for liquigel ibuprofen 400 mg, acetaminophen 1000 mg, ketoprofen 25 mg, and placebo in the treatment of postoperative dental pain. J Clin Pharmacol 41:1238–1247
Henry D, Lim LL, Garcia Rodriguez LA, Perez Gutthann S, Carson JL, Griffin M, Savage R, Logan R, Moride Y, Hawkey C, Hill S, Fries JT (1996) Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. Br Med J 312:1563–1566
Albuquerque AFM, Fonteles CSR, do Val DR, Chaves HV, Bezerra MM, Pereira KMA, de Barros Silva PG, de Lima BB, Soares ECS, Ribeiro TR, Costa FWG (2017) Effect of pre-emptive analgesia on clinical parameters and tissue levels of TNF-α and IL-1β in third molar surgery: a triple-blind, randomized, placebo-controlled study. Int J Oral Maxillofac Surg 46:1615–1625
Figueroba SR, Groppo MF, Faibish D, Groppo FC (2018) The action of anti-inflammatory agents in healthy temporomandibular joint synovial tissues is sex-dependent. Int J Oral Maxillofac Surg 47:205–213
Yamashita Y, Sano N, Shimohira D, Danjo A, Goto M (2014) A parallel-group comparison study of celecoxib with loxoprofen sodium in third mandibular molar extraction patients. Int J Oral Maxillofac Surg 43:1509–1513
Chan FK, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL (2010) Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet 376:173–179
Inchingolo F, Tatullo M, Marrelli M, Inchingolo AM, Picciariello V, Inchingolo AD, Dipalma G, Vermesan D, Cagiano R (2010) Clinical trial with bromelain in third molar exodontia. Eur Rev Med Pharmacol Sci 14:771–774
Lixuan Z, Jingcheng D, Wengin Y, Jianhua H, Baojun L, Xiaotao F (2010) Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models. Pulm Pharmacol Ther 23:411–419
Wang H, Zhang L, Jiang N, Wang Z, Chong Y, Fu F (2013) Anti-inflammatory effects of escin are correlated with the glucocorticoid receptor/NF-κB signaling pathway, but not the COX/PGF2α signaling pathway. Exp Ther Med 6:419–422
Meiser P, Xu Z, Kirsch G, Jacob C (2014) Chapter 18 – systemic enzyme therapy: fact or fiction? A review with focus on bromelains, proteolytic enzymes from the pineapple plant. In: Jacob C (ed) Recent advances in redox active plant and microbial products. Springer Science+Business Media, Dordrecht, pp 449–467
Bormann KH, Weber K, Kloppenburg H, Koch A, Meiser P, Gellrich NC (2016) Perioperative bromelain therapy after wisdom teeth extraction - a randomized, placebo-controlled, double-blinded, three-armed, cross-over dose-finding study. Phytother Res 30:2012–2019
Chou TC, Chang LP, Li CY, Wong CS, Yang SP (2003) The antiinflammatory and analgesic effects of baicalin in carrageenan-evoked thermal hyperalgesia. Anesth Analg 97:1724–1729
Matarese G, Ramaglia L, Cicciù M, Cordasco G, Isola G (2017) The effects of diode laser therapy as an adjunct to scaling and root planing in the treatment of aggressive periodontitis: a 1-year randomized controlled clinical trial. Photomed Laser Surg 35:702–709
Wang T, Fu F, Zhang L, Han B, Zhu M, Zhang X (2009) Effects of escin on acute inflammation and the immune system in mice. Pharmacol Rep 61:697–704
Barbalho JC, Vasconcellos RJ, de Morais HH, Santos LA, Almeida RA, Rêbelo HL, Lucena EE, de Araújo SQ (2017) Effects of co-administered dexamethasone and nimesulide on pain, swelling, and trismus following third molar surgery: a randomized, triple-blind, controlled clinical trial. Int J Oral Maxillofac Surg 46:236–242
Lokken P, Olsen I, Bruaset I, Norman-Pedersen K (1975) Bilateral surgical removal of impacted lower third molar teeth as a model for drug evaluation: a test with ibuprofen. Eur J Clin Pharmacol 8:209–216
Isola G, Matarese G, Lo Giudice G, Briguglio F, Alibrandi A, Crupi A, Cordasco G, Ramaglia L (2017) A new approach for the treatment of lateral periodontal cysts with an 810-nm diode laser. Int J Periodontics Restorative Dent 37:e120–e129
Al-Khateeb TH, Nusair Y (2008) Effect of the proteolytic enzyme serrapeptase on swelling, pain and trismus after surgical extraction of mandibular third molars. Int J Oral Maxillofac Surg 37:264–268
Deferm JT, Schreurs R, Baan F, Bruggink R, Merkx MAW, Xi T, Bergé SJ, Maal TJJ (2018) Validation of 3D documentation of palatal soft tissue shape, color, and irregularity with intraoral scanning. Clin Oral Investig 22:1303–1309
Ristow O, Hohlweg-Majert B, Stürzenbaum SR, Kehl V, Koerdt S, Hahnefeld L, Pautke C (2014) Therapeutic elastic tape reduces morbidity after wisdom teeth removal--a clinical trial. Clin Oral Investig 18:1205–1212
Ferlazzo N, Currò M, Zinellu A, Caccamo D, Isola G, Ventura V, Carru C, Matarese G, Ientile R (2017) Influence of MTHFR genetic background on p16 and MGMT methylation in oral squamous cell Cancer. Int J Mol Sci 18:E724. https://doi.org/10.3390/ijms18040724
Costa FW, Soares EC, Esses DF, Silva PG, Bezerra TP, Scarparo HC, Ribeiro TR, Fonteles CS (2015) A split-mouth, randomized, triple-blind, placebo-controlled study to analyze the pre-emptive effect of etoricoxib 120 mg on inflammatory events following removal of unerupted mandibular third molars. Int J Oral Maxillofac Surg 44:1166–1174
De Menezes SA, Cury PR (2010) Efficacy of nimesulide versus meloxicam in the control of pain, swelling and trismus following extraction of impacted lower third molar. Int J Oral Maxillofac Surg 39:580–584
Bjornsson GA, Haanaes HR, Skoglund LA (2003) Ketoprofen 75 mg qid versus acetaminophen 1000 mg qid for 3 days on swelling, pain and other postoperative events after third molar surgery. J Clin Pharmacol 43:305–314
Martuscelli R, Toti P, Sbordone L, Guidetti F, Ramaglia L, Sbordone C (2014) Five-year outcome of bone remodelling around implants in the maxillary sinus: assessment of differences between implants placed in autogenous inlay bone blocks and in ungrafted maxilla. Int J Oral and Maxillofac Surg 43:1117–1126
Isola G, Cicciù M, Fiorillo L, Matarese G (2017) Association Between Odontoma and Impacted Teeth. J Craniofac Surg 28:755–758
Funding
This work was carried out with funding by the Department of Biomedical, Odontostomatological Sciences and of Morphological and Functional Images, University of Messina.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the manuscript and took responsibility for the integrity of the data that is present in this study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study followed the Declaration of Helsinki on medical protocol, and the institutional review board of the University of Messina, Messina, Italy, approved the study protocol (09/17).
Informed consent
Written informed consent was obtained from the patient for publication. A copy of the written consent may be requested for review from the corresponding author.
Electronic supplementary material
ESM 1
(DOC 221 kb)
Rights and permissions
About this article
Cite this article
Isola, G., Matarese, M., Ramaglia, L. et al. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: a randomized, triple-blind, controlled clinical trial. Clin Oral Invest 23, 2443–2453 (2019). https://doi.org/10.1007/s00784-018-2690-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2690-9