Abstract
Objective
The aim of this study was to compare the clinical and radiographic efficacy of guided tissue regeneration with a modified perforated collagen membrane (MPM) or standard collagen membrane (CM) in the treatment of intrabony defects in patients with aggressive periodontitis (AgP).
Materials and methods
Fifteen AgP patients were included in the study. Two single intrabony defects of at least 3 mm depth with ≥ 6 mm probing pocket depth (PPD) from each patient were randomly assigned to either xenogenic graft plus MPM (test group) or xenogenic graft plus CM (control group). PPD, clinical attachment level (CAL), and gingival recession (GR) were recorded at baseline and at 12 months. The radiographic assessments included the measurements of defect depth (DD), change in alveolar crest position (ACP), linear defect fill (LDF), and percentage defect fill (%DF).
Results
After treatment, PPD, CAL, DD, and ACP values improved significantly in both groups, without statistical differences between them. However, with respect to LDF and %DF, the 12-month radiographic analysis at MPM-treated sites showed a significant improvement compared to the 6-month outcomes, that was not observed at control sites (additional LDF of 0.4 ± 0.5 mm, p = 0.010 and %DF of 6.4 ± 7.6%, p = 0.025).
Conclusions
Both strategies proved effective in the treatment of intrabony defects in patients with AgP. Nonetheless, enhanced LDF and %DF 12 months postoperatively at MPM-treated sites may stem from cellular and molecular migration from the periosteum and overlying gingival connective tissue through barrier’s pores.
Clinical relevance
Modification of CM may have positive ramifications on periodontal regeneration.
Similar content being viewed by others
Introduction
Aggressive periodontitis (AgP) is characterized by a rapid destruction of tooth-supporting structures leading to formation of intrabony defects in conjunction with relatively low plaque levels in individuals who are systematically healthy [1]. Its treatment consists of brief non-surgical phase and following surgeries.
Periodontal regeneration is a demanding procedure that requires restoration of root cementum, alveolar bone, and periodontal ligament fibers simultaneously with appropriate integration among all regenerated types of tissues. It may be achieved by placing barrier membrane over intrabony defect to prevent ingrowth of connective and epithelial cells during the initial wound healing phase [2]. Bioresorbable collagen membranes (CM) are frequently used for this purpose [3]. Albeit guided tissue regeneration (GTR) is a well-established procedure, the treatment of intrabony defects, especially in patients with AgP, remains a challenge and novel therapeutic strategies are very much needed.
Oral-periosteum plays a pivotal role in bone endogenous repair processes and its cambium serves as unlimited source of cells that possess a great expansion capacity and stemness. Periosteum-derived progenitor cells (PDPCs) are self-committed to differentiate in vitro towards osteoblast lineage, without any osteogenic medium to support their differentiation [4]. The high osteogenic potential of jaw periosteal cells, especially MSCA-1+ fraction, is closely related to the expression of lipoprotein receptor-related protein 6 (LRP-6), stanniocalcin 1 (STC-1), and tissue inhibitor of metalloproteinases-4 (TIMP-4) [5]. Periosteal cell populations modify their potential with age, with a significant increase in IL-6 mRNA expression and receptor activator of nuclear factor kappa-B ligand/osteoprotegerin ratio [6]. In vivo studies indicated that PDPCs are indispensable for bone graft healing and remodeling [7].
Likewise, human gingival tissue contains numerous types of cells with a remarkable regenerative potential. Gingival fibroblasts were found to express variable levels of mRNA for alkaline phosphate and bone morphogenetic protein 2/4 (BMP2/4) in vitro, which demonstrated that appropriate stimuli may direct their capacity of hard tissue formation [8]. By the same token, gingival mesenchymal stem/progenitor cells (GMSCs), with self-renewal capabilities, remarkable differentiation potential, long-term telomerase expression, and outstanding immunomodulatory properties have been isolated [9, 10]. GMCSs preconditioned in an osteogenic differentiation medium demonstrated on the mRNA level expression of bone specific markers, including Runx2, collagen I, collagen III, ALP, osteonectin (ON), osteopontin (OP), osteocalcin (OC), and osterix [9]. GMSCs showed few inflammation-related changes when incubated with proinflammatory mediators (TNF-α, IL-1β), which suggested good response to unfavorable environmental conditions [10]. A bone regenerative capacity of GMSCs has been confirmed by multiple studies, as these cells were able to repair the critical size mandibular/calvarial defects in animals [11,12,13]. Moreover, the transplanted GMSCs proved a significant periodontal regenerative potential in vivo, with newly formed alveolar bone, cementum, and periodontal ligament fibers [14,15,16].
The abovementioned studies shed new light on the classical periodontal compartmentalization theory advocating that the gingiva does not contribute to periodontal regeneration. However, during GTR procedure, CM is put over intrabony defect, excluding any input of GMSCs and PDPCs on healing processes taking place in intrabony defect. The present study was conducted to compare the clinical and radiographic outcomes of the use of modified perforated collagen membranes (MPM) that allow cellular and molecular migration with the use of standard CM in the treatment of localized intrabony defects in patients with AgP.
Material and methods
Study design and subject population
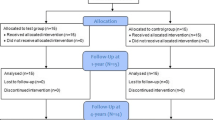
The study was planned as a randomized, prospective, double-blinded, split-mouth, controlled clinical trial. The investigators who assessed the clinical and radiographic outcomes were not aware of the identity of the treatment protocol. From each patient, two comparative (as similar as possible) intrabony defects were selected and subsequently surgically treated by GTR procedures in a 2-week time span. The study was carried out in accordance with the Helsinki Declaration of 1975, as revised in Tokyo in 2004 after approval of the study design by the Bioethics Committee of Medical University of Warsaw (KB/135/2014). The subject population was recruited among patients with a provisional diagnosis of AgP referred to the Department of Periodontology of Medical University of Warsaw by their general dentists. Patients were thoroughly re-examined at a dedicated clinic by a specialist, and if diagnosis of AgP was confirmed and patients fulfilled the inclusion criteria, they were allocated for meticulous non-surgical periodontal treatment including oral hygiene instructions, full-mouth scaling, and root debridement in conjunction with systemic antibiotics of amoxicillin 500 mg + metronidazole 250 mg three times per day for 1 week. The patients were reevaluated at 6 weeks and thoroughly informed of the nature, potential risks, and benefits of their participation in the study. All patients signed an informed consent form before enrollment (Fig. 1).
Sample size calculation
According to a study by Gamal and Iacono, using MPM in GTR of intrabony defects may result in additional gain of approximately 1.2 mm for clinical attachment level (CAL) when compared to CM [17]. Consequently, the sample size calculation determined that eight subjects per treatment group would provide 80% power to disclose a true difference of 1.2 mm between test and control, assuming 1.0 mm as the common standard deviation and 0.05 as the level of significance. However, considering that some patients could be lost during follow-up, 15 patients were enrolled.
Inclusion and exclusion criteria
All patients were diagnosed with AgP in line with the 1999 American Academy of Periodontology classification [1]. The inclusion criteria were as follows: (1) no systematic medical compromising conditions; (2) no medications affecting periodontal status; (3) only light smokers were included (< 10 cigarettes/day); (4) not pregnant or lactating; (5) familial aggregation (history of periodontitis in parents or siblings); (6) presence of at least two teeth with probing pocket depth (PPD) ≥ 6 mm and CAL ≥ 5 mm associated with an intrabony defect of at least 3 mm as detected in diagnostic periapical radiographs; (7) full mouth plaque score (FMPS) ≤ 20%; (8) bleeding on probing score (BoP) ≤ 20%; (9) teeth had to be vital or properly treated; (10) no furcation involvement of the teeth presenting the intraosseous defects; (11) the width of keratinized tissue on the facial aspect of the selected teeth ≥ 2 mm.
Randomization and allocation concealment
Two defects were selected from each patient and randomly assigned before surgical appointments. For allocation, a computerized random number generator was used by a person not involved in the study. Allocation to treatment intervention was concealed in sealed and opaque envelopes and was revealed to the surgeon during the procedure. No information on treatment allocation was provided to the patient.
Clinical measurements
Clinical parameters were assessed by the same experienced and calibrated examiner (MZ). A total of 6 non-study patients with AgP were recruited for the calibration exercise. The designated examiner recorded full-mouth PPD and CAL with an interval of 24 h between recordings. Calibration was accepted when ≥ 90% of the recordings could be reproduced within a difference of 1.0 mm and an exact agreement was repeated in 75% of measurements. The following clinical parameters were evaluated using a graded periodontal probe (UNC probe 15 mm, Hu-Friedy) and rounded off to the nearest millimeter: (1) FMPS was recorded as the percentage of total surfaces (four aspects per tooth) that revealed the presence of plaque [18], (2) bleeding on probing (BoP) was assessed dichotomously at six points per tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual) [19], (3) PPD was defined as the distance from the gingival margin to the base of the periodontal pocket at six points per tooth, (4) CAL was determined as the distance from the cemento-enamel junction (CEJ) or from the most apical extension of restoration/crown, when present, to the base of the pocket at six points per tooth, and (5) gingival recession (GR) as the distance from the gingival margin to CEJ at the mid-buccal point of a tooth. PPD, CAL, and GR were evaluated immediately before surgery and 12 months postoperatively, while FMPS and BoP were assessed at baseline, at 1, 3, 6, and 12 months post-surgery.
Surgical treatment
The surgical procedures were performed by one surgeon (BG) in accordance with principles of minimally invasive surgical technique [20]. The defects were meticulously debrided and the roots were carefully planned. Subsequently, the required quantity of the deproteinized bovine bone allograft (DBBA, Bio-Oss®, Geistlich Biomaterials, Princeton, New Jersey, USA) was delivered in small increments into the defects. Once a defect was filled, an envelope containing the randomized treatment assignment was opened and the treatment option was appointed to either covering the defect with modified perforated porcine collagen membrane (MPM/test, Bio-Gide®, Geistlich Biomaterials) or placing standard collagen membrane (CM/control, Bio-Gide®). Membrane perforations were prepared just before its placement as described previously by using a custom-made acrylic template, leaving a coronal occlusive rim of ~ 3 mm [17]. This was followed by manually perforating ~ 0.46- to 0.72-mm-diameter round holes (with a standard hand-spreader number 40, Poldent, Warsaw, Poland) at a distance of 2 mm throughout the length of the membrane. Subsequently, membranes were trimmed according to the template prepared for each defect and adapted in line with the protocol of the manufacturer. The suturing approach consisted of a single modified internal mattress sutures (5/0 polypropylene monofilament suture, Prolene 5/0 16 mm 3/8, Ethicon, Somerville, New Jersey, USA) in the inter-dental areas and simple passing sutures in vertical incisions.
Clinical characterization of the intrabony defect
Defect morphology was characterized intra-surgically in terms of the total depth (distance measured from the most coronal point of the bony walls surrounding the defect to the deepest point in the defect) and width of the defect (distance between the most coronal point of the bony walls surrounding the defect to the root surface) as described previously [21]. The defects were described as 1-, 2-, and 3-wall or combination defects.
Postoperative period
The patients received meticulous postoperative instructions and were asked to avoid brushing, flossing, and chewing in the treated area for a period of 2 weeks. Prescription included ibuprofen 600 mg at the end of surgery and every 6 h in case of pain, and 0.2% chlorhexidine mouth-rinsing three times daily for 3 weeks. At week 2, sutures were removed and patients resumed careful brushing with a soft toothbrush. Patients were placed on a 2-week recall system for 3 months and every 3 months for 1 year.
Radiographic assessments
Radiographic examination was performed preoperatively, 6 and 12 months postoperatively by an experienced and calibrated clinician (SJ) who was masked with respect to the surgical intervention. An intra-examiner calibration was previously done by examining 12 non-study-related radiographs. Periapical radiographs were taken using the paralleling technique using an x-ray unit operating at 70 kV, 4 mA, and 0.1-s exposure time with the aid of individual film-holder device consisting of a bite block to achieve identical film placement at each evaluation. The radiographs were digitized and analyzed using Planmeca Romexis Viewer software (Planmeca, Helsinki, Finland). The anatomical landmarks were selected on the radiographs based on the criteria set by Schei et al. [22], which included CEJ, alveolar crest (AC), and base of the defect (BD). The most apical extension of an interproximal restoration, when present, was used instead of CEJ. The most coronal area where the periodontal ligament maintained an even width was identified as the most apical extent of intrabony defect [23]. An auxiliary line was drawn in tooth axis (AUX1) and a secondary line (AUX2) from AC, perpendicular to AUX1. The distance from the spot where AUX2 crossed the CEJ-BD line to the base of the defect was measured as defect depth (DD) (Fig. 2). The length between CEJ and AC was assessed as alveolar crest position (ACP). The radiographic defect angle of each defect was measured between the CEJ-BD line of the involved tooth and the bone defect surface [24, 25]. Linear defect fill (LDF) was calculated by subtracting CEJ-BD distance at the end of 12 months from CEJ-BD at baseline, while percentage defect fill (%DF) by dividing LDF by DD at baseline [26].
Statistical analysis
Statistical analysis was carried out with R 3.3.1 software [R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org]. The main outcome variable was CAL gain at 12 months, and the secondary variables were PPD reduction, LDF, and %DF at 12 months. The measurements at the site with the most advanced attachment loss at baseline were used for statistical analysis. The 12-month changes in clinical outcomes were calculated by subtracting the 12-month values from the baseline values. Consequently, a positive 12-month change signified a reduction in PPD, a gain in CAL, and a decrease in GR. Normality of distribution was assessed using Shapiro-Wilk test and by visual inspection of histograms. Comparisons between treated and non-treated sites at the same time points, as well as comparison between two time points in one study group, were performed using paired Wilcoxon test. To compare measurements in three time points (baseline, 6, 12 months), Friedman test was used. If Friedman test provided a significant difference, the individual groups were compared by paired Wilcoxon test. Bonferroni correction was adopted to avoid multiple comparison problem. Descriptive statistics were presented as mean and standard deviation (95% confidence interval). Any p values of less than 0.05 (p < 0.05) were considered statistically significant.
Results
Fifteen patients (10 women and 5 men, aged 22–49; mean age 37.9 ± 7.95 years) were enrolled in the study, and each was treated with both MPM and CM GTR surgery in 2 weeks’ time span. All subjects completed the 6- and 12-month follow-up. Patients demonstrated good oral hygiene standards. At baseline, FMPS was 8.4% (± 7.6), while BoP 12.6% (± 7.3). Both treatment groups had similar clinical and radiographic parameters prior to treatment, as well as defects morphology evaluated intra-surgically (Table 1).
Healing was uneventful in all patients. Complete gingival wound closure was accomplished for all defect sites. Nonetheless, membrane exposure was observed at 2 to 3 weeks after surgery in three of the MPM-treated sites and two of the CM-treated sites. Exposed areas were irrigated with a 0.2% chlorhexidine solution at the follow-up visits and application of 1% chlorhexidine gel was administered daily until complete re-epithelialization.
Twelve months after treatment, PPD and CAL values statistically significantly improved in both groups, while the GR values increased as shown in (Table 1). There were no statistical differences in assessed probing measurements after surgeries between the MPM- and CM-treated sites (Table 1).
Analysis of radiographs carried out 6 and 12 months postoperatively showed a statistically significant reduction in DD values in both groups (Table 2). However, only in the test group, decrease in DD in 6–12-month observation period was significant. Twelve months after treatment, ACP values decreased significantly in both groups (Table 2). There was a decrease trend in values of ACP in 0–6 and 6–12 periods, but after application of the Bonferroni correction, these changes did not reach statistical significance.
A comparison between groups affirmed that, between 6- and 12-month observation period, the MPM test group showed a significant improvement in DD reduction compared with the CM-treated sites (Fig. 3). There were no statistical differences between the mean control and test sites in LDF and %DF values at 6 and 12 months postoperatively (Table 3). However, the 12-month radiographic analysis at the test sites showed a significant improvement compared to the 6-month outcomes in both LDF and %DF, that was not observed at control sites (Table 3).
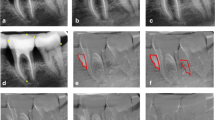
a, b, c Radiographs of test site (MPM-treated). Intrabony defect on distal surface of tooth 36. a Baseline radiograph. b Radiograph at 6 months post-surgery. c Radiograph at 12 months post-surgery. Decrease in radiographic defect depth and width as compare between 6 and 12 months postoperatively. d, e, f Radiographs of control site (OM-treated). Intrabony defect on mesial surface of tooth 16. a Baseline radiograph. b Radiograph at 6 months post-surgery. c Radiograph at 12 months post-surgery. Lack of changes in radiographic defect depth and width as compare between 6 and 12 months postoperatively
Discussion
The present study evaluates the use of collagen membranes modified with permeable pores (MPM) in the treatment of intrabony defects in patients with AgP and compares its outcomes with standard CM, which completely isolated the periosteum and gingival connective tissue elements from the defect area. It reveals that both treatment modalities were equally effective with respect to clinical and radiographic outcomes. In addition to surgical technique, patient compliance and a strict maintenance regimen appear to be key elements. At 12 months for MPM- and CM-treated sites, the mean CAL gain was 4.7 mm (± 2.1) and 4.3 mm (± 1.3), respectively, while the mean PPD reduction was 4.0 mm (± 2.1) and 3.5 mm (± 1.2) and %DF 87.1% (± 10.8) and 82.3% (± 11.7), respectively. Significant differences between the two groups could not be demonstrated. Interestingly, a significantly higher gain of LDF and %DF between 6 and 12 months after treatment at the test sites was noted. During this period, LDF and %DF gain for MPM-treated sites was 0.4 mm (± 0.5) and 6.4% (± 7.6), while for CM-treated sites 0.0 mm (± 0.2) and 0.2% (± 3.8), respectively. It indicates that bone regeneration is still an ongoing process at 6 months postoperatively, which is of utmost importance [27, 28].
These results may be attributed to the design of MPM, which were composed of a dense occlusive collar that inhibited epithelial downgrowth and the perforated body that permitted both cellular and molecular migration allowing more physiologic interplays between different elements of the periodontium. Gamal and Iacono implied that the migration process may compensate the limited cellularity of the periodontal defects; hence, the intrabony defects covered by traditional CM seemed to be deprived of a great supply of progenitor cells and biologic mediators [17]. Periodontal wound healing is a complex physiological process with well-orchestrated series of biological events. Osteogenesis relies on a sequential gene activation, starting with BMP2 pathway which triggers mRNA Runx2 transcription [29]. BMP2 is involved in all crucial osteogenic pathways: Wnt/β-catenin cascade, fibroblast growth factor-2 (FGF2), Hedgehog (Hh) signaling as well as in Smad and Notch signaling [30]. Translocation of gingival fibroblasts/GMSCs and PDPCs alters their behavior in response to periodontal ligament and bone mediators [31, 32]. Manifold molecular interactions may guide the activation and subsequent differentiation of these cells towards an osteoblastic lineage.
Clinical and radiographic improvements in the present study are in agreement with the conclusions of a systematic review showing that GTR may be successfully performed in patients affected by AgP [32]. Surprisingly, only two RCT studies (three articles) have been reported in the literature and none of the biomaterials used could be affiliated with better outcomes. In Queiroz et al.’s study, the use of a non-resorbable barrier resulted in similar changes to those obtained in patients in which the bone substitute was mixed with P-15 without any membrane [33]. In Rakmanee et al.’s research, both clinical and radiographic results showed substantial comparability between GTR procedures and access flap therapies [27, 34]. To the best of our knowledge, the concept of using MPM in patients with AgP has not been tested yet.
Gamal and Iacono used MPM for GTR of intrabony defects in patients with chronic periodontitis [17]. The postoperative differences between the two groups were 3.9 mm (± 1.1) and 3.0 mm (± 0.6) for PPD reduction; 3.3 mm (± 0.8) and 2.1 mm (± 0.9) for CAL gain; − 3 mm (± 0.5) and − 3.1 mm (± 0.9) for DBL at 6 months, respectively, in favor of the MPM-treated sites, which is in line with our results. However, in our study, xenogenic DBBA was added to maintain the volume of the filled defects. In further investigations, MPM coverage of periodontal defects was associated with a significant increase of BMP2 concentration and growth factor upregulation (vascular endothelial growth factor VEGF, platelet-derived growth factor-BB PDGF-BB) in gingival crevicular fluid during the early postoperative period [35, 36].
The effects of barrier’s pore size on cellular migration and membrane properties were recently evaluated. Membrane perforations may mechanically interlock fibrin strands, hence securing supracrestal blood clot. Moreover, integration of membrane pores with gingival connective tissue from one side and with clot from the opposing side could help to stabilize the flap. Macro-membrane perforations of 0.2, 0.4, and 0.7 mm could preserve membrane stiffness and allow for cellular migration [31]. However, they rendered total gingival tissue isolation inexecutable. In our study, perforation diameters varied from about 0.46 to 0.72 mm.
Our research has some plausible limitations. First, it does not verify the true nature of healing, as histological evaluation was not performed due to ethical concerns. Second, only 15 individuals were enrolled in the project with 12-month observation period, whereas the paucity of studies on GTR in patients affected by AgP makes the thorough evaluation of our findings futile. Third, there were relatively more maxillary defects in the control group and in three patients maxillary defects were compared with mandibular defects. Albeit from each patient two intrabony defects (as similar as possible) were selected and randomly assigned to the surgical protocol, different distribution of defects according to the tooth position (maxillary/mandibular tooth) may influence the results and undermine the generalizability of the present findings. In the light of these facts, there is a need for further long-term clinical trials with bigger sample size to verify the present findings. More investigations are also required to determine perforations’ diameter that would be permeable for regenerative cellular and molecular elements, while being obstructive for unwanted gingival connective tissue components. Greater insight of biological mechanisms involved in specific gingival stem cells capacity to participate in true periodontal regeneration is needed, before predictable use of MPM becomes clinical reality. Nonetheless, it seems that a successful GTR approach can be achieved in AgP patients.
Conclusions
GTR technique using modified perforated collagen membrane or traditional collagen membrane achieved comparable clinical and radiographic outcomes in patients with AgP. However, the modified membranes provided enhanced treatment outcomes in terms of radiographic linear defect fill and % defect fill 12 months postoperatively. In conclusion, within the limits of our study, the present findings may imply that:
-
Both GTR with MPM or CM can be successfully implemented for the treatment of intrabony defects of AgP patients
-
Both treatment strategies resulted in significant gain in CAL and reduction in PPD at 12 months, as well as in significant radiographic DD reduction and radiographic defect fill of the intrabony components at 6 and 12 months
-
The GTR sites with MPM showed ongoing significant radiographic defect fill of the intrabony defects from 6 to 12 months
References
Lang NP, Bartold PM, Cullinan M, Jeffcoat M, Mombelli A, Murakami S, Page R, Papapanou P, Tonetti M, Van Dyke T (1999) Consensus report: aggressive periodontitis. Ann Periodontol 4(1):53. https://doi.org/10.1902/annals.1999.4.1.53
Nyman S, Gottlow J, Karring T, Lindhe J (1982) The regenerative potential of the periodontal ligament. J Clin Periodontol 9(3):257–265. https://doi.org/10.1111/j.1600-051X.1982.tb02065.x
Sheikh Z, Qureshi J, Alshahrani AM, Nassar H, Ikeda Y, Glogauer M, Gauss B (2017) Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology 105(1):1–12. https://doi.org/10.1007/s10266-016-0267-0
Ceccarelli G, Graziano A, Benedetti L, Imbriani M, Romano F, Ferrarotti F, Aimetti M, Cusella de Angelis GM (2016) Osteogenic potential of human oral-periosteal cells (PCs) isolated from different oral origin: an in vitro study. J Cell Physiol 231(3):607–612. https://doi.org/10.1002/jcp.25104
Olbrich M, Rieger M, Reinert S, Alexander D (2012) Isolation of osteoprogentiors from human jaw periosteal cells: a comparison of two magnetic separation methods. PLoS One 7(10):e47176. https://doi.org/10.1371/journal.pone.0047176
Ferretti C, Lucarini G, Andreoni C, Salvolini E, Bianchi N, Vozzi G, Gigante A, Mattioli-Belmonte M (2015) Human periosteal derived stem cell potential: the impact of age. Stem Cell Rev 11(3):487–500. https://doi.org/10.1007/s12015-014-9559-3
Zhang X, Award HA, O'Keefe RJ, Guldberg RE, Schwarz EM (2008) A perspective: engineering periosteum for structural bone graft healing. Clin Orthop Relat Res 466(8):1777–1787. https://doi.org/10.1007/s11999-008-0312-6
Ivanoceski S, Li H, Haase H, Bartold P (2001) Expression of bone associated macromolecules by gingival and periodontal ligament fibroblasts. J Periodontal Res 36(3):131–141. https://doi.org/10.1034/j.1600-0765.2001.360301.x
Fawzy El-Sayed KM, Dörfer CE (2016) Gingival mesenchymal stem/progenitor cells: a unique tissue engineering gem. Stem Cells Int 7154327:1–16. https://doi.org/10.1155/2016/7154327
Santamaria S, Sanchez N, Sanz M, Garcia-Sanz JA (2017) Comparison of periodontal ligament and gingiva-derived mesenchymal stem cells for regenerative therapies. Clin Oral Invest 21(4):1095–1102. https://doi.org/10.1007/s00784-016-1867-3
Moshaverinia A, Chen C, Xu X, Akiyama K, Ansari S, Zadeh HH, Shi S (2014) Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng Part A 20:611–621. https://doi.org/10.1089/ten.TEA.2013.0229
Xu QC, Wang ZG, Ji QX, Yu XB, Xu XY, Yuan CQ, Deng J, Yang PS (2014) Systematically transplanted human gingiva-derived mesenchymal stem cells contributing to bone tissue regeneration. Int J Clin Exp Pathol 7(8):4922–4929
Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W, Yang P, Pei X (2011) Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev 20(12):2093–2102. https://doi.org/10.1089/scd.2010.0523
Fawzy El-Sayed KM, Paris S, Becker ST, Neuschl M, De Buhr W, Sälzer S, Wulff A, Elrefai M, Darhous MS, El-Masry M, Wiltfang J, Dörfer CE (2012) Periodontal regeneration employing gingival margin-derived stem/progenitor cells: an animal study. J Clin Periodontol 39(9):861–870. https://doi.org/10.1111/j.1600-051X.2012.01904.x
Yu X, Ge S, Chen S, Xu Q, Zhang J, Guo H, Yang P (2013) Human gingiva-derived mesenchymal stromal cells contribute to periodontal regeneration in beagle dogs. Cells Tissues Organs 198(6):428–437. https://doi.org/10.1159/000360276
Fawzy El-Sayed KM, Mekhemar MK, Beck-Broichsitter BE, Bähr T, Hegab M, Receveur J, Heneweer C, Becker ST, Wiltfang J, Dörfer CE (2015) Periodontal regeneration employing gingival margin-derived stem/progenitor cells in conjunction with IL-1ra hydrogel synthetic extracellular matrix. J Clin Periodontol 42(5):448–457. https://doi.org/10.1111/jcpe.12401
Gamal AY, Iacono VJ (2013) Enhancing guided tissue regeneration of periodontal defects by using a novel perforated barrier membrane. J Peridontol 284(7):905–913. https://doi.org/10.1902/jop.2012.120301
O'Leary TJ, Drake RB, Naylor JE (1972) The plaque control record. J Periodontol 43(1):38. https://doi.org/10.1902/jop.1972.43.1.38
Ainamo J, Bay I (1975) Problems and proposal for recording gingivitis and plaque. Int Dent J 25(4):229–235
Cortellini P, Tonetti MS (2007) A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra-bony defects: a novel approach to limit morbidity. J Clin Periodontol 34(1):87–93. https://doi.org/10.1111/j.1600-051X.2006.01020.x
Cortellini P, Pini-Prato GP, Tonetti MS (1993) Periodontal regeneration of human intrabony defects. II. Re-entry procedures and bone measures. J Periodontol 64(4):261–268. https://doi.org/10.1902/jop.1993.64.4.261
Schei O, Waerhaug J, Lovdal A, Arro A (1959) Alveolar bone loss as related to oral hygiene and age. J Periodontol 30(1):7–16. https://doi.org/10.1902/jop.1959.30.1.7
Björn H, Halling A, Thyberg H (1969) Radiographic assessment of marginal bone loss. Odontol Revy 20:165–179
Tonetti M, Pini-Prato G, Cortellini P (1993) Periodontal regeneration of human infrabony defects. IV. Determinants of the healing response. J Periodontol 64:934–940
Eickholz P, Hörr T, Klein Fm Hassfeld S, Kim T-S (2004) Radiographic parameters for prognosis of periodontal healing of intrabony defects: two different definitions of defect depth. J Periodontol 75(1):399–407. https://doi.org/10.1111/j.1600-051X.2006.01020.x
Jayakumar A, Rajababu P, Rohini S, Butchibabu K, Naveen A, Reddy PK, Vidyasagar S, Satyanarayana D, Pavan Kumar S (2011) Multi-center, randomized clinical trial on efficacy and safety of recombinant human platelet-derived growth factor with ß-tricalcium phosphate in human intra-osseous periodontal defects. J Clin Periodontol 38(2):163–172. https://doi.org/10.1111/j.1600-051X.2010.01639.x
Rakmanee T, Griffiths GS, Auplish G, Darbar U, Petrie A, Olsen I, Donos N (2016) Radiographic outcomes following treatment of intrabony defect with guided tissue regeneration in aggressive periodontitis. Clin Oral Investig 20(6):1227–1235. https://doi.org/10.1007/s00784-015-1609-y
Wenzel A, Warrer K, Karring T (1992) Digital subtraction radiography in assessing bone changes in periodontal defects following guided tissue regeneration. J Clin Periodontol 19:208–213
Chung JE, Park JH, Yun JW, Kang YH, Park BW, Hwang SC, Cho YC, Sung IY, Woo DK, Byun JH (2016) Cultured human periosteum-derived cells can differentiate into osteoblasts in a peroxisome proliferator-activated receptor gamma-mediated fashion via bone morphogenetic protein signaling. Int J Med Sci 13(11):806–818. https://doi.org/10.7150/ijms.16484
Chappous V, Gamer L, Cox K, Lowery JW, Bosshardt DD, Rosen V (2012) Periosteal BMP2 activity drives bone graft healing. Bone 51(4):800–809. https://doi.org/10.1016/j.bone.2012.07.017
Gamal AY, Al-Berry NN, Hassan AA, Rashed LA, Iacono VJ (2017) In vitro evaluation of the human gingival fibroblast/gingival mesenchymal stem cell dynamics through perforated guided tissue membranes: cell migration, proliferation and membrane stiffness assay. J Periodontal Res 52(3):628–635. https://doi.org/10.1111/jre.12431
Corbella S, Weinstein R, Francetti L, Taschieri S, Del Fabbro M (2016) Periodontal regeneration in aggressive periodontitis patients: a systemic review of literature. J Investig Clin Dent. https://doi.org/10.1111/jicd.12245
Queiroz AC, Nobrega PB, Oliveira FS, Novaes AB Jr, Taba M Jr, Palioto DB, Grisi MF, Souza SL (2013) Treatment of intrabony defects with anorganic bone matrix/p-15 or guided tissue regeneration in patients with aggressive periodontitis. Braz Dent J 24(3):204–212. https://doi.org/10.1590/0103-6440201302169
Rakmanee T, Griffiths GS, Auplish G, Darbar U, Petrie A, Olsen I, Donos N (2016) Treatment of intrabony defects with guided tissue regeneration in aggressive periodontitis: clinical outcomes at 6 and 12 months. Clin Oral Investig 20(6):1217–1225. https://doi.org/10.1007/s00784-015-1608-z
Gamal AY, Abdel-Ghaffar KA, Iacono VJ (2016) Gingival crevicular fluid vascular endothelial cell growth factor and platelet-derived growth factor-BB release profile following the use of perforated barrier membranes during treatment of intrabony defects: a randomized clinical trial. J Periodontal Res 51(3):407–416. https://doi.org/10.1111/jre.12321
Gamal AY, Aziz M, Salama MH, Iacono VJ (2014) Gingival crevicular fluid bone morphogenetic protein-2 release profile following the use of perforated membrane barriers in localized intrabony defects: a randomized clinical trial. J Int Acad Perodontol 16:55–63
Funding
The study was self-supported by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was reviewed and approved by the Bioethics Committee of Medical University of Warsaw, Poland (KB/135/2014).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Górski, B., Jalowski, S., Górska, R. et al. Treatment of intrabony defects with modified perforated membranes in aggressive periodontitis: a 12-month randomized controlled trial. Clin Oral Invest 22, 2819–2828 (2018). https://doi.org/10.1007/s00784-018-2368-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2368-3