Abstract
Objectives
The aim of our study is to prove and validate the existence of an osteogenic progenitor cell population within the human maxillary Schneiderian sinus membrane (hMSSM) and to demonstrate their potential for bone formation.
Materials and methods
Ten hMSSM samples of approximately 2 × 2 cm were obtained during a surgical nasal approach for treatment of chronic rhinosinusitis and were retained for this study. The derived cells were isolated, cultured, and assayed at passage 3 for their osteogenic potential using the expression of Alkaline phosphatase, alizarin red and Von Kossa staining, flow cytometry, and quantitative real-time polymerase chain reaction.
Results
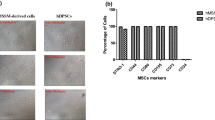
hMSSM-derived cells were isolated, showed homogenous spindle-shaped fibroblast-like morphology, characteristic of mesenchymal progenitor cells (MPCs), and demonstrated very high expression of MPC markers such as STRO-1, CD44, CD90, CD105, and CD73 in all tested passages. In addition, von Kossa and Alizarin red staining showed significant mineralization, a typical feature of osteoblasts. Moreover, alkaline phosphatase (ALP) activity was significantly increased at days 7, 14, 21, and 28 of culture in hMSSM-derived cells grown in osteogenic medium, in comparison to controls. Furthermore, osteogenic differentiation significantly upregulated the transcriptional expression of osteogenic markers such as ALP, Runt-related transcription factor 2 (Runx-2), bone morphogenetic protein (BMP)-2, osteocalcin (OCN), osteonectin (ON), and osteopontin (OPN), confirming that hMSSM-derived cells are of osteoprogenitor origin. Finally, hMSSM-derived cells were also capable of producing OPN proteins upon culturing in an osteogenic medium.
Conclusion
Our data showed that hMSSM holds mesenchymal osteoprogenitor cells capable of differentiating to the osteogenic lineage.
Clinical relevance
hMSSM contains potentially multipotent postnatal stem cells providing a promising clinical application in preimplant and implant therapy.








Similar content being viewed by others
References
Boyne PJ, James RA (1980) Grafting of the maxillary sinus floor with autogenous marrow and bone. J of Oral Surg 38:613–616
Tatum H Jr (1986) Maxillary and sinus implant reconstructions. Dent Clin N Am 30:207–229
Misch CE (1987) Maxillary sinus augmentation for endosteal implants: organized alternative treatment plans. Int J of Oral Impl 4:49–58
Santagata M, Tozzi U, Tartaro G, et al. (2014) Maxillary sinus augmentation with autologous and heterologous bone graft: a clinical and radiographic report of immediate and delayed implant placement. J Maxillofac Oral Surg 13:401–408
Miron, RJ, Zhang Q, Sculean A et al. (2016) Osteoinductive potential of 4 commonly employed bone grafts. Clin Oral Investig 2016
Del Fabio M, Testori T (2009) Anatomy of the maxillary sinus. In: Testori T, Del Fabio M, Weinstein R, Wallace S (eds) Maxillary sinus surgery, 1st edn. Quintessence Publishing Co, Ltd, Berlin, pp 7–22
Froum SJ, Wallace SS, Elian N, Cho SC, Tarnow DP (2006) Comparison of mineralized cancellous bone allograft (puros) and anorganic bovine bone matrix (bio-Oss) for sinus augmentation: histomorphometry at 26 to 32 weeks after grafting. Int J Periodont Restor Dent 26:543–551
Ohayon L (2014) Maxillary sinus floor augmentation using biphasic calcium phosphate: a histologic and histomorphometric study. Int J Oral Maxillofac Impl 29:1143–1148
Sehn FP, Dias RR, de Santana Santos T (2015) Fresh-frozen allografts combined with bovine bone mineral enhance bone formation in sinus augmentation. J Biomater Appl 29:1003–1013
Alayan J, Vaquette C, Farah C, Ivanovski S (2015) A histomorphometric assessment of collagen-stabilized anorganic bovine bone mineral in maxillary sinus augmentation—a prospective clinical trial. Clin Oral Implants Res. doi:10.1111/clr.12694
Lutz R, Berger-Fink S, Stockmann P, Neukam FW, Schlegel KA (2015) Sinus floor augmentation with autogenous bone vs. a bovine-derived xenograft—a 5-year retrospective study. Clin Oral Implants Res 26:644–648
Meloni SM, Jovanovic SA, Lolli FM, et al. (2015) Grafting after sinus lift with anorganic bovine bone alone compared with 50 :50 anorganic bovine bone and autologous bone : results of a pilot randomised trial at one year. Br J Oral Maxillofac Surg 53:436–441
Moon JW, Sohn DS, Heo JU, Kim JS (2015) Comparison of two kinds of bovine bone in maxillary sinus augmentation: a histomorphometric study. Implant Dent 24:19–24
Chen TW, Chang HS, Leung KW, Lai YL, Kao SY (2007) Implant placement immediately after the lateral approach of the trap door window procedure to create a maxillary sinus lift without bone grafting: a 2-year retrospective evaluation of 47 implants in 33 patients. J Oral Maxillofac Surg 65:2324–2328
Hatano N, Sennerby L, Lundgren S (2007) Maxillary sinus augmentation using sinus membrane elevation and peripheral venous blood for implant-supported rehabilitation of the atrophic posterior maxilla: case series. Clin Implant Dent Relat Res 9:150–155
Sohn DS, Moon JW, Lee WH (2011) Comparison of new bone formation in the maxillary sinus with and without bone grafts: immunochemical rabbit study. Int J Oral Maxillofac Implants 26:1033–1042
Lin IC, Gonzalez AM, Chang HJ, Kao SY, Chen TW (2011) A 5-year follow-up of 80 implants in 44 patients placed immediately after the lateral trap-door window procedure to accomplish maxillary sinus elevation without bone grafting. Int J Oral Maxillofac Implants 26:1079–1086
Riben C, Thor A (2015) Follow-up of the sinus membrane elevation technique for maxillary sinus implants without the use of graft material. Clin Implant Dent Relat Res. doi:10.1111/cid.12360
Ducy P, Schinke T, Karsenty G (2001) The osteoblast: a sophisticated fibroblast under central surveillance. Science 289:1501–1504
Bianco P, Riminucci M, Gronthos S, Robey PG (2001) Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180–192
Bianco P, Sacchetti B, Riminucci M (2011) Osteoprogenitors and the hematopoietic microenvironment. Best Pract Res Clin Haematol 24:37–47
Mouraret S, Von Kaeppler E, Bardet C (2014) The potential for vertical bone regeneration via maxillary periosteal elevation. J Clin Periodontol 41:1170–1177
Zhao J, Wang R (2015) Biologic characteristics and osteogenic differentiation of maxillary primordium mesenchymal cells. J Craniofac Surg 26:340–342
Jung S, Kleineidam B, Kleinheinz J (2015) Regenerative potential of human adipose-derived stromal cells of various origins. J Craniomaxillofac Surg 43:2144–2151
Bianco P, Robey PG (2015) Skeletal stem cells. Development 142:1023–1027
Wang P, Xie F, Pan J, Tang X (2012) Differences in the structure and osteogenesis capacity of the periosteum from different parts of minipig mandibles. J Oral Maxillofac Surg 70:1331–1337
Gothard D, Greenhough J, Ralph E, Oreffo RO (2014) Prospective isolation of human bone marrow stromal cell subsets: a comparative study between Stro-1-, CD146- and CD105-enriched populations. J Tissue Eng 18:5
Yan XZ, Both SK, Yang PS et al. (2014) Human periodontal ligament derived progenitor cells: effect of STRO-1 cell sorting and Wnt3a treatment on cell behavior. Biomed Res Int 145423. doi: 10.1155/2014/145423
Pittenger MF, Mackay AM, Beck SC, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Bayat M, Momen Heravi F, Mahmoudi M, Bahrami N (2015) Bone reconstruction following application of bone matrix gelatin to alveolar defects: a randomized clinical trial. Int J Organ Transplant Med 6:176–181
Lin GH, Lim G, Chan HL, Giannobile WV, Wang HL (2015) Recombinant human bone morphogenetic protein 2 outcomes for maxillary sinus floor augmentation: a systematic review and meta-analysis. Clin Oral Implants Res. doi:10.1111/clr.12737
Kelly MP, Vaughn OL, Anderson PA (2015) Systematic review and meta-analysis of recombinant human bone morphogenetic protein-2 in localized alveolar ridge and maxillary sinus augmentation. J Oral Maxillofac Surg
Seo SJ, Bark CW, Lim JH, Kim YG (2015) Bone dynamics in the upward direction after a maxillary sinus floor elevation procedure: serial segmentation using synchrotron radiation micro-computed tomography. Int J Nanomedicine 10 Spec Iss:129–136
Gruber R, Kandler B, Fuerst G, Fischer MB, Watzek G (2004) Porcine sinus mucosa holds cells that respond to bone morphogenetic protein (BMP)-6 and BMP-7 with increased osteogenic differentiation in vitro. Clin Oral Implants Res 15:575–580
Kim SW, Lee IK, Yun KI, Kim CH, Park JU (2009) Adult stem cells derived from human maxillary sinus membrane and their osteogenic differentiation. Int J Oral Maxillofac Implants 24:991–998
Srouji S, Kizhner T, Ben David D, et al. (2009) The Schneiderian membrane contains osteoprogenitor cells: in vivo and in vitro study. Calcif Tissue Int 84:138–145
Yun KI, Kim DJ, Park JU (2013) Osteogenic potential of adult stem cells from human maxillary sinus membrane by simvastatin in vitro: preliminary report. J Korean Assoc Oral Maxillofac Surg 39:150–155
Cho KS, Park HY, Roh HJ, et al. (2014) Human ethmoid sinus mucosa: a promising novel tissue source of mesenchymal progenitor cells. Stem Cell Res Ther 24:5–15
Lie N, Merten HA, Meyns J, et al. (2015) Elevation of the maxillary sinus membrane for de-novo bone formation: first results of a prospective study in humans. J Craniomaxillofac Surg 43:1670–1677
Bensaha T, El Mjabber H (2016) Evaluation of new bone formation after sinus augmentation with two different methods. Int J Oral Maxillofac Surg 45:93–98
Srouji S, Ben-David D, Lotan R, Riminucci M, Livne E, Bianco P (2010) The innate osteogenic potential of the maxillary sinus (Schneiderian) membrane: an ectopic tissue transplant model simulating sinus lifting. Int J Oral Maxillofac Surg 39:793–801
Shi S, Gronthos S (2003) Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18:696–704
Xu J, Li Z, Hou Y, Fang W (2015) Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am J Transl Res 7(12):2527–2535 eCollection 2015
Ram VS, Parthiban SU, Mithradas N, Prabhakar R (2015) Bone biomarkers in periodontal disease: a review article. J Clin Diagn Res 9:ZE07–ZE10. doi:10.7860/JCDR/2015/11268.5438
Zoch ML, Clemens TL, Riddle RC (2016) New insights into the biology of osteocalcin. Bone 82:42–49. doi:10.1016/j.bone.2015.05.046
Mangano FG, Colombo M, Veronesi G, Caprioglio A, Mangano C (2015) Mesenchymal stem cells in maxillary sinus augmentation: a systematic review with meta-analysis. World J Stem Cells 7(6):976–991. doi:10.4252/wjsc. v7.i6.976
Gronthos S, Zannettino AC, Hay SJ, et al. (2003) Molecular and cellular characterization of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 116:1827–1835
Lin NH, Gronthos S, Bartold PM (2009) Stem cells and future periodontal regeneration. Periodontology 51:239–251
Seo BM, Miura M, Gronthos S, et al. (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S (2005) The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res 8:191–199
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci 97:13625–13630
Cicconetti A, Sacchetti B, Bartoli A, et al. (2007) Human maxillary tuberosity and jaw periosteum as sources of osteoprogenitor cells for tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104(5):618e1–61812
Acknowledgments
This work was supported by grants from Lebanese University (18840).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The work was supported by a grant from the Lebanese University (18840) to AB and Lebanese NCSR grant to KZ, Beirut, Lebanon.
Ethical approval
This study was approved by the Institutional Review Board of the Lebanese University (CUEMB1/2014—18840). The protocol is registered in the Clinical Trial.gov (ID NCT02676921).
All experiments were conducted in compliance with current good clinical practice standards and in accordance with relevant guidelines and regulations and the principles set forth under the Declaration of Helsinki (1989).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Figure 9
Establishment and characterization of adherent spindle shaped hMSSM derived cells in culture (10× magnifications). A total of 7 samples of primary human maxillary sinus Shneiderian membrane (MSSM) cultures are shown for various passages (P0, P1, P2, and P3). Photographs were taken at Day 5 of culture for each passage.(GIF 532 kb)
Rights and permissions
About this article
Cite this article
Berbéri, A., Al-Nemer, F., Hamade, E. et al. Mesenchymal stem cells with osteogenic potential in human maxillary sinus membrane: an in vitro study. Clin Oral Invest 21, 1599–1609 (2017). https://doi.org/10.1007/s00784-016-1945-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-1945-6