Abstract
Objectives
Bisphosphonates and denosumab are antiresorptive drugs used for the treatment of osteoporosis and oncological tumors. A severe side effect is osteonecrosis of the jaw. Monocyte/macrophage dysfunction is considered to play a distinct role in osteonecrosis. THP-1 monocytic cells were used in this study to elucidate the influence of zoledronate and denosumab on phorbol-12-myrisate-13-acetate (PMA)-induced macrophage differentiation and function in real-time.
Materials and methods
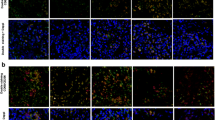
Macrophagic differentiation of the THP-1 suspension cells was measured by cell adherence in the presence or absence of different concentrations of zoledronate (0.5, 5, 50 μM) and denosumab (1, 10, 20, 40 μg/mL) using the real-time xCELLigence system. Additionally, a live/dead staining was performed by fluorescence microscopy.
Results
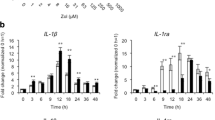
THP-1 cells demonstrated a regular initial PMA-induced differentiation to macrophages by live measurements of cell adherence and by an increase in CD68 surface expression as detected by flow cytometry. The addition of zoledronate led to cell detachment of the THP-1-derived macrophages in a dose-dependent manner in contrast to denosumab. Cell detachment was based on cell death as confirmed by live/dead staining, revealing elevated numbers of dead cells following addition of high zoledronate concentrations. However, denosumab did not deteriorate THP-1 cell viability.
Conclusion
Our results demonstrate that zoledronate but not denosumab suppresses monocytic THP-1 cell viability after macrophagic differentiation dose-dependently.
Clinical relevance
This is the first real-time study providing evidence for a dose-dependent immunosuppressive effect of zoledronate in contrast to denosumab on local macrophages.







Similar content being viewed by others
References
Baron R, Ferrari S, Russell RG (2011) Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48:677–692
Hoefert S, Eufinger H (2005) Osteonecrosis of the jaws as a possible adverse effect of the use of bisphosphonates. Mund Kiefer Gesichtschir 9:233–238
Moreau MF, Guillet C, Massin P, Chevalier S, Gascan H, Basle MF et al (2007) Comparative effects of five bisphosphonates on apoptosis of macrophage cells in vitro. Biochem Pharmacol 73:718–723
Russell RG (2011) Bisphosphonates: the first 40 years. Bone 49:2–19
Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA et al (2012) Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 48:3082–3092
Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK et al (2012) Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov 11:401–419
Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH et al (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 28:5132–5139
Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J et al (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29:1125–1132
Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L et al (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377:813–822
Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B (2009) American association of oral and maxillofacial surgeons position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J Oral Maxillofac Surg 67(5):2–12
Saad F, Brown JE, Van PC, Ibrahim T, Stemmer SM, Stopeck AT et al (2012) Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol 23:1341–1347
Hewitt RE, Lissina A, Green AE, Slay ES, Price DA, Sewell AK (2005) The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol 139:101–111
Ferrari-Lacraz S, Ferrari S (2011) Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos Int 22:435–446
Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61:1115–1117
Diz P, Lopez-Cedrun JL, Arenaz J, Scully C (2012) Denosumab-related osteonecrosis of the jaw. J Am Dent Assoc 143:981–984
Ruggiero SL (2013) An office-based approach to the diagnosis and management of osteonecrosis. Atlas Oral Maxillofac Surg Clin North Am 21:167–173
Carmagnola D, Canciani E, Sozzi D, Biglioli F, Moneghini L, Dellavia C (2013) Histological findings on jaw osteonecrosis associated with bisphosphonates (BONJ) or with radiotherapy (ORN) in humans. Acta Odontol Scand 71:1410–1417
Favia G, Pilolli GP, Maiorano E (2009) Histologic and histomorphometric features of bisphosphonate-related osteonecrosis of the jaws: an analysis of 31 cases with confocal laser scanning microscopy. Bone 45:406–413
Kumar V, Sinha RK (2013) Evolution and etiopathogenesis of bisphosphonates induced osteonecrosis of the jaw. N Am J Med Sci 5:260–265
Jones AC, Sedghizadeh PP (2014) Bisphosphonate-related osteonecrosis of the jaws is caused by dental procedures that violate oral epithelium; this is no longer a mysterious disease. Oral Surg Oral Med Oral Pathol Oral Radiol 117:392–393
Kunzmann V, Bauer E, Wilhelm M (1999) Gamma/delta T-cell stimulation by pamidronate. N Engl J Med 340:737–738
Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT et al (2005) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. 2004. J Bone Miner Res 20:2275–2282
Burr DB, Allen MR (2009) Mandibular necrosis in beagle dogs treated with bisphosphonates. Orthod Craniofac Res 12:221–228
Walter C, Al-Nawas B, Frickhofen N, Gamm H, Beck J, Reinsch L et al (2010) Prevalence of bisphosphonate associated osteonecrosis of the jaws in multiple myeloma patients. Head Face Med 6:11. doi:10.1186/1746-160X-6-11
Yoneda T, Hagino H, Sugimoto T, Ohta H, Takahashi S, Soen S et al (2010) Bisphosphonate-related osteonecrosis of the jaw: position paper from the allied task force committee of Japanese society for bone and mineral research, Japan osteoporosis society, Japanese society of periodontology, Japanese society for oral and maxillofacial radiology, and Japanese society of oral and maxillofacial surgeons. J Bone Miner Metab 28:365–383
Qi WX, Tang LN, He AN, Yao Y, Shen Z (2014) Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol 19:403–410
Pazianas M (2011) Osteonecrosis of the jaw and the role of macrophages. J Natl Cancer Inst 103:232–240
Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K (1980) Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 26:171–176
Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH (2010) The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 1:e8668
Plattner VE, Ratzinger G, Engleder ET, Gallauner S, Gabor F, Wirth M (2009) Alteration of the glycosylation pattern of monocytic THP-1 cells upon differentiation and its impact on lectin-mediated drug delivery. Eur J Pharm Biopharm 73:324–330
Urcan E, Haertel U, Styllou M, Hickel R, Scherthan H, Reichl FX (2010) Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent Mater 26:51–58
Block GA, Bone HG, Fang L, Lee E, Padhi D (2012) A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 27:1471–1479
Hoefert S, Schmitz I, Weichert F, Gaspar M, Eufinger H (2014) Macrophages and bisphosphonate-related osteonecrosis of the jaw (BRONJ): evidence of local immunosuppression of macrophages in contrast to other infectious jaw diseases. Clin Oral Investig. doi:10.1007/s00784-014-1273-7
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661
Jeganathan S, Fiorino C, Naik U, Sun HS, Harrison RE (2014) Modulation of osteoclastogenesis with macrophage M1- and M2-inducing stimuli. PLoS ONE 8:e104498. doi: 10.1371
Roelofs AJ, Stewart CA, Sun S, Blazewska KM, Kashemirov BA, McKenna CE et al (2012) Influence of bone affinity on the skeletal distribution of fluorescently labeled bisphosphonates in vivo. J Bone Miner Res 27:835–847
Rogers MJ, Chilton KM, Coxon FP, Lawry J, Smith MO, Suri S et al (1996) Bisphosphonates induce apoptosis in mouse macrophage-like cells in vitro by a nitric oxide-independent mechanism. J Bone Miner Res 11:1482–1491
Kuiper JW, Forster C, Sun C, Peel S, Glogauer M (2012) Zoledronate and pamidronate depress neutrophil functions and survival in mice. Br J Pharmacol 165:532–539
Hagelauer N, Pabst AM, Ziebart T, Ulbrich H, Walter C (2014) In vitro effects of bisphosphonates on chemotaxis, phagocytosis, and oxidative burst of neutrophil granulocytes. Clin Oral Investig. doi:10.1007/s00784-014-1219-0
Orsini G, Failli A, Legitimo A, Adinolfi B, Romanini A, Consolini R (2011) Zoledronic acid modulates maturation of human monocyte-derived dendritic cells. Exp Biol Med (Maywood) 236:1420–1426
Cheng ML, Fong L (2014) Effects of RANKL-Targeted Therapy in Immunity and Cancer. Front Oncol 329. doi: 10.3389/fonc.2013.00329
Seshasayee D, Wang H, Lee WP, Gribling P, Ross J, van BN et al (2004) A novel in vivo role for osteoprotegerin ligand in activation of monocyte effector function and inflammatory response. J Biol Chem 279:30202–30209
Acknowledgments
This study was financially supported by the German Society of Dentistry and Oral Medicine (Deutsche Gesellschaft für Zahn-, Mund- und Kieferheilkunde e.V.; DGZMK).
Conflicts of interest
We authors declare no other financial relationships with the organisation that sponsored the research, other organisations, nor other conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoefert, S., Hoefert, C.S., Albert, M. et al. Zoledronate but not denosumab suppresses macrophagic differentiation of THP-1 cells. An aetiologic model of bisphosphonate-related osteonecrosis of the jaw (BRONJ). Clin Oral Invest 19, 1307–1318 (2015). https://doi.org/10.1007/s00784-014-1358-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-014-1358-3