Abstract
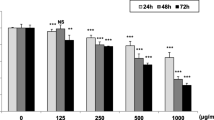
Betel quid (BQ) chewing is a common oral habit in South Asia and Taiwan. BQ consumption may increase the risk of oral squamous cell carcinoma (OSCC), oral submucous fibrosis (OSF), and periodontitis as well as systemic diseases (atherosclerosis, hypertension, etc.). However, little is known about the toxic effect of BQ components on endothelial cells that play important roles for angiogenesis, carcinogenesis, tissue fibrosis, and cardiovascular diseases. EAhy 926 (EAHY) endothelial cells were exposed to arecoline, a major BQ alkaloid, for various time periods. Cytotoxicity was estimated by 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide assay. The cell cycle distribution of EAHY cells residing in sub-G0/G1, G0/G1, S-, and G2/M phases was analyzed by propidium iodide staining of cellular DNA content and flow cytometry. Some EAHY cells retracted, became round-shaped in appearance, and even detached from the culture plate after exposure to higher concentrations of arecoline (> 0.4 mM). At concentrations of 0.4 and 0.8 mM, arecoline induced significant cytotoxicity to EAHY cells. At similar concentrations, arecoline induced G2/M cell cycle arrest and increased sub-G0/G1 population, a hallmark of apoptosis. Interestingly, prolonged exposure to arecoline (0.1 mM) for 12 and 21 days significantly suppressed the proliferation of EAHY cells, whereas EAHY cells showed adaptation and survived when exposed to 0.05 mM arecoline. These results suggest that BQ components may contribute to the pathogenesis of OSF and BQ chewing-related cardiovascular diseases via toxicity to oral or systemic endothelial cells, leading to impairment of vascular function. During BQ chewing, endothelial damage may be induced by areca nut components and associate with the pathogenesis of OSF, periodontitis, and cardiovascular diseases.





Similar content being viewed by others

References
Jeng JH, Chang MC, Hahn LJ (2001) Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol 37:477–492
Gupta PC, Ray CS (2004) Epidemiology of betel quid usage. Ann Acad Med Singapore 33(4 Suppl):31–36
Zhang X, Reichart PA (2007) A review of betel quid chewing, oral cancer and precancer in Mainland China. Oral Oncol 43:424–430
IARC (2004) Betel-quid and areca-nut chewing and some areca-nut-derived nitrosamines. In: IARC monographs on the evaluation of carcinogenic risks to humans, vol 85. IARC, World Health Organization, Lyon, France
Nelson BS, Heischober B (1999) Betel nut: a common drug used by naturalized citizens from India, Far East Asia, and the South Pacific Islands. Ann Emerg Med 34:238–243
Ko YC, Chiang TA, Chang SJ, Hsieh SF (1992) Prevalence of betel quid chewing habit in Taiwan and related sociodemographic factors. J Oral Pathol Med 21:261–264
Trivedy CR, Craig G, Warnakulasuriya S (2002) The oral health consequences of chewing areca nut. Addict Biol 7:115–125
Jeng JH, Chang MC (2007) Betel quid chewing and cardiovascular diseases: a traditional oral habit with potential new health issues. Atherosclerosis Commentary, September
Lin WY, Chiu TY, Lee LT, Lin CC, Huang CY, Huang KC (2008) Betel nut chewing is associated with increased risk of cardiovascular disease and all-cause mortality in Taiwanese men. Am J Clin Nutr 87:1204–1211
Yen AM, Chen LS, Chiu YH, Boucher BJ, Chen TH (2008) A prospective community-population-registry based cohort study of the association between betel-quid chewing and cardiovascular disease in men in Taiwan. Am J Clin Nutr 87:70–78
Fang CY, Han WN, Fong DY (2000) A morphometric study on the microvessel in oral submucous fibrosis. Hunan Yi Ke Da Xue Bao 25:55–57
Desai RS, Mamatha GS, Khatri MJ, Shetty SJ (2010) Immunohistochemical expression of CD34 for characterization and quantification of mucosal vasculature and its probable role in malignant transformation of atrophic epithelium in oral submucous fibrosis. Oral Oncol 46:553–558
Arjungi KN (1976) Areca nut: a review. Arzneimittelforschung 26:951–956
Nair J, Ohshima H, Friesen M, Croisy A, Bhide SV, Bartsch H (1985) Tobacco-specific and betel nut-specific N-nitroso compounds: occurrence in saliva and urine of betel quid chewers and formation in vitro by nitrosation of betel quid. Carcinogenesis 6:295–303
Jeng JH, Kuo ML, Hahn LJ, Kuo MY (1994) Genotoxic and non-genotoxic effects of betel quid ingredients on oral mucosal fibroblasts in vitro. J Dent Res 73:1043–1049
Jeng JH, Ho YS, Chan CP et al (2000) Areca nut extract up-regulates prostaglandin production, cyclooxygenase-2 mRNA and protein expression of human oral keratinocytes. Carcinogenesis 21:1365–1370
Chang MC, Wu HL, Lee JJ et al (2004) The induction of prostaglandin E2 production, interleukin-6 production, cell cycle arrest, and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK activation. J Biol Chem 279:50676–50683
Van Wyk CW, Olivier A, Miranda CM, Bijl P, Grobler-Rabie AF (1994) Observations on the effect of areca nut extracts on oral fibroblast proliferation. J Oral Pathol Med 23:145–148
Lee PH, Chang MC, Chang WH et al (2006) Prolonged exposure to arecoline arrested human KB epithelial cell growth: regulatory mechanisms of cell cycle and apoptosis. Toxicology 220:81–89
Jeng JH, Chen SY, Liao CH et al (2002) Modulation of platelet aggregation by areca nut and betel leaf ingredients: roles of reactive oxygen species and cyclooxygenase. Free Radic Biol & Med 32:860–871
Edgell CJ, Mcdonald CC, Graham JB (1983) Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 80:3734–3737
Edgell CJ, Haizlip JE, Bagnell CR, Packenham JP, Harrison P, Wilbourn B, Madden VJ (1990) Endothelium specific Weibel–Palade bodies in a continuous human cell line, EA.hy926. In Vitro Cell Dev Biol 26:1167–1172
Staton CA, Brown NJ, Lewis CE (2003) The role of fibrinogen and related fragments in tumour angiogenesis and metastasis. Expert Opin Biol Ther 3:1105–1120
Aranda E, Owen GI (2009) A semi-quantitative assay to screen for angiogenic compounds and compounds with angiogenic potential using the EA.hy926 endothelial cell line. Biol Res 42:377–389
Jeng JH, Lu FJ, Hahn LJ, Wang YJ, Kuo MYP (1994) Eugenol triggers different pathobiological effects on oral mucosal fibroblasts in vitro. J Dent Res 73:1050–1055
Chang MC, Chen LI, Chan CP et al (2010) The role of reactive oxygen species and hemeoxygenase-1 expression in the cytotoxicity, cell cycle alteration and apoptosis of dental pulp cells induced by BisGMA. Biomaterials 31:8164–8171
Iwakiri Y, Groszmann RJ (2007) Vascular endothelial dysfunction in cirrhosis. J Hepatol 46:927–934
Balakumar P, Chakkarwar VA, Krishan P, Singh M (2009) Vascular endothelial dysfunction: a tug of war in diabetic nephropathy? Biomed Pharmacother 63:171–179
Sitla S, Tomasoni L, Atzeni F et al (2010) From endothelial dysfunction to atherosclerosis. Autoimmun Rev 9:830–834
Kahaleh B (2008) The microvascular endothelium in scleroderma. Rheumatology 47(Suppl 5):v14–v15
Cox S, Vickers ER, Ghu S, Zoellner H (2010) Salivary arecoline levels during areca nut chewing in human volunteers. J Oral Pathol Med 39:465–469
Zeng Q, Lagunoff D, Masaracchia R, Goeckeler Z, Cote G, Wysolmerski R (2000) Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J Cell Sci 113:471–482
Garcia JG, Verin AD, Schaphorst KL (1996) Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemost 22:309–315
Onoda JM, Kartak SS, Diglio CA (1999) Radiation induced endothelial cell retraction in vitro correlation with acute pulmonary edema. Pathol Oncol Res 5:49–55
Jeng JH, Wang YJ, Chiang BL et al (2003) Roles of keratinocyte inflammation in oral cancer: regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis 24:1301–1315
Offenbacher S, Beck JD (2005) A perspective on the potential cardioprotective benefits of periodontal therapy. Am Heart J 149:950–954
Boucher BJ, Mannan N (2002) Metabolic effects of the consumption of Areca catechu. Addict Biol 7:103–110
Wess J, Lambrecht G, Moser U, Mutschler E (1987) Stimulation of ganglionic muscarinic M1 receptors by a series of tertiary arecaidine and isoarecaidine esters in the pithed rat. Eur J Pharmacol 134:61–67
Vander B, Van Eyk AD, Van Wyk CW, Stander IA (2001) Diffusion of reduced arecoline and arecaidine through human vaginal and buccal mucosa. J Oral Pathol Med 30:200–205
Harvey W, Scutt A, Meghji S, Canniff JP (1986) Stimulation of human buccal fibroblasts in vitro by betel-nut alkaloids. Arch Oral Biol 31:45–49
Giri S, Idle JR, Chen C, Zabriskie TM, Krausz KW, Gonzalez FJ (2006) A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse. Chem Res Toxicol 19:818–827
Giri S, Krausz KW, Idle JR, Gonzalez FJ (2007) The metabolomics of (+/−)-arecoline 1-oxide in the mouse and its formation by human flavin-containing monooxygenases. Biochem Pharmacol 73:561–573
Wu IC, Chen PH, Wang CJ et al (2010) Quantification of blood betel quid alkaloids and urinary 8-hydroxydeoxyguanosine in humans and their association with betel chewing habits. J Anal Toxicol 34:325–331
Hu CW, Chang YZ, Wang HW, Chao MR (2010) High-throughput simultaneous analysis of five urinary metabolites of areca nut and tobacco alkaloids by isotope-dilution liquid chromatography–tandem mass spectrometry with on-line solid-phase extraction. Cancer Epidemiol Biomarkers Prev 19:2570–2581
Elledge S (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274:1664–1672
Thangjam GS, Kondaiah P (2009) Regulation of oxidative-stress responsive genes by arecoline in human keratinocytes. J Periodont Res 44:673–682
Lin CC, Chang MC, Chang HH et al (2009) Areca nut-induced micronuclei and cytokinesis failure in Chinese hamster ovary cells is related to reactive oxygen species production and actin filament deregulation. Environ Mol Mutagen 50:367–374
Chang MC, Ho YS, Lee PH et al (2001) Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis 22:1527–1535
Tsai YS, Lee KW, Huang JL et al (2008) Arecoline, a major alkaloid of areca nut, inhibits p53, represses DNA repair, and triggers DNA damage response in human epithelial cells. Toxicology 249:230–237
Chou WW, Guh JY, Tsai JF et al (2008) Arecoline-induced growth arrest and p21WAF1 expression are dependent on p53 in rat hepatocytes. Toxicology 243:1–10
Wang YC, Tsai YS, Huang JL et al (2010) Arecoline arrests cells at prometaphase by deregulating mitotic spindle assembly and spindle assembly checkpoint; implication for carcinogenesis. Oral Oncol 46:255–262
Nojima H (2006) Protein kinases that regulate chromosome stability and their downstream targets. Genome Dyn 1:131–148
Acknowledgements
This study is supported by a grant from National Science Council (NSC-97-2320-B-255-001-MY3), Taiwan, Republic of China and Chang Gung Memorial Hospital.
Conflicts of interest
The authors declare that there are no conflicts of interest for this submission.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mei-Chi Chang and Ming-Lun Hsu made an equal contribution to the first author.
Rights and permissions
About this article
Cite this article
Tseng, SK., Chang, MC., Su, CY. et al. Arecoline induced cell cycle arrest, apoptosis, and cytotoxicity to human endothelial cells. Clin Oral Invest 16, 1267–1273 (2012). https://doi.org/10.1007/s00784-011-0604-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-011-0604-1