Abstract
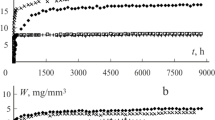
This study investigated the diffusion kinetics of a nanofilled (Filtek Z350) and a midifill (Filtek P60) resin composite immersed in distilled water, artificial saliva and lactic acid. Resin composite specimens were desiccated, immersed in the media, weighed at suitable time intervals until they reached sorption equilibrium and were then desiccated again. Sorption and solubility (µg/mm3) were calculated based on ISO 4049:2000(E). The diffusion coefficient (m2.s−1) was determined according to Flick’s second law. The degree of conversion (DC%) was evaluated by FT-IR and the action of the media on the surfaces of the resin composite was evaluated by SEM. Z350 immersed in lactic acid presented the highest sorption (25.9 ± 1.3). The highest solubility was presented by Z350 immersed in lactic acid (5.6 ± 0.9), followed by P60 immersed in lactic acid (4.4 ± 0.5). The other groups presented no significant difference among them. The diffusion coefficients of both resin composites immersed in lactic acid and that of Z350 immersed in artificial saliva were significantly higher. The lowest diffusion coefficient was presented by P60 immersed in distilled water. The DC% was not significant, (p > 0.05). The SEM analysis showed that the effect of lactic acid on the resin composites was more deleterious than those of water and artificial saliva.




Similar content being viewed by others
References
Curtis AR, Shortall AC, Marquis PM, Palin WM (2008) Water uptake and strength characteristics of a nanofilled resin-based composite. J Dent 36:186–193
Sideridou ID, Karabela MM, Vouvoudi EC (2008) Dynamic thermomechanical properties and sorption characteristics of two commercial light cured dental resin composites. Dent Mater 24:737–743
Gonçalves L, Noronha Filho JD, Guimarães JGA, Poskus LT, Silva EM (2008) Solubility, salivary sorption and degree of conversion of dimethacrylate-based polymeric matrixes. J Biomed Mater Res B Appl Biomater 85:320–325
Zhang Y, Xu J (2008) Effect of immersion in various media on the sorption, solubility, elution of unreacted monomers, and flexural properties of two model dental composite compositions. J Mater Sci Mater Med 19:2477–2483
Silva EM, Almeida GS, Poskus LT, Guimarães JGA (2008) Relationship between the degree of conversion, solubility and salivary sorption of a hybrid and a nanofilled resin composite: influence of the light-activation mode. J Appl Oral Sci 16:161–166
Toledano M, Osorio R, Osorio E, Fuentes V, Prati C, Garcia-Godoy F (2003) Sorption and solubility of resin-based restorative dental materials. J Dent 31:43–50
Sideridou I, Tserki V, Papanastasiou G (2003) Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 24:655–665
Hofmann N, Renner J, Hugo B, Klaiber B (2002) Elution of leachable components from resin composite after plasma arc vs. standard or soft-start halogen light irradiation. J Dent 30:223–232
Kalachandra S, Wilson TW (1992) Water sorption and mechanical properties of light-cured proprietary composite tooth restorative materials. Biomaterials 13:105–109
Söderholm KJ, Mukherjee R, Longmate J (1996) Filler leachability of composites stored in distilled water or artificial saliva. J Dent Res 75:1692–1699
Musanje L, Darvell BW (2003) Aspects of water sorption from the air, water and artificial saliva in resin composite restorative materials. Dent Mater 19:414–422
Rawls HR, Esquivel-Upshaw J (2005) Resinas restauradoras. In: Anusavice KJ (ed) Phillips materiais dentários, 3rd edn. Elsevier, Rio de Janeiro, pp 375–418
Mitra SB, Wu D, Holmes BN (2003) An application of nanotechnology in advanced dental materials. J Am Dent Assoc 134:1382–1390
Beun S, Glorieux T, Devaux J, Vreven J, Leloup G (2007) Characterization of nanofilled compared to universal and microfilled composites. Dent Mater 23:51–59
Rodrigues SAJ, Zanchi CH, Carvalho RV, Demarco FF (2007) Flexural strength and modulus of elasticity of different types of resin-based composites. Braz Oral Res 21:16–21
Dickens SH, Stansbury JW, Choi KM, Floyd CJE (2003) Photopolymerization kinetics of methacrylate dental resins. Macromolecules 36:6043–6053
Schneider LFJ, Consani S, Ogliari F, Correr AB, Sobrinho LC, Sinhoreti MAC (2006) Effect of time and polymerization cycle on the degree of conversion of a resin composite. Oper Dent 31:489–495
Santos C, Clarke RL, Braden M, Guitian F, Davy KWM (2002) Water absorption characteristics of dental composites incorporating hydroxyapatite filler. Biomaterials 23:1897–1904
Asaoka K, Hirano S (2003) Diffusion coefficient of water through dental composite resin. Biomaterials 24:975–979
Palin WM, Fleming GJP, Burke FJT, Marquis PM, Randall RC (2005) The influence of short and medium-term water immersion on the hydrolytic stability of novel low-shrink dental composites. Dent Mater 21:852–863
Lagouvardos PE, Pissis P, Kyritsis A, Daoukaki D (2003) Water sorption and water-induced molecular mobility in dental composite resins. J Mater Sci Mater Med 14:753–759
Nicholson JW, Millar BJ, Czarnecka H, Limanowska-Shaw H (1999) Storage of Polyacid-modified resin composites (“compomers”) in lactic acid solution. Dent Mater 15:413–416
Asmussen E (1984) Softening o BISGMA-based polymers by ethanol and by organic acids of plaque. Scand J Dent Res 92:257–261
De Gee AJ, Wendt SL, Werner A, Davidson CL (1996) Influence of enzymes and plaque acids on in vitro wear of dental composites. Biomaterials 17:1327–1332
Bagheri R, Tyas MJ, Burrow MF (2007) Subsurface degradation of resin-based composites. Dent Mater 23:944–951
Distler W, Kröncke A (1983) The acid pattern in human dental plaque. J Dent Res 62:87–91
Geddes DA (1975) Acids produced by human dental plaque metabolism in situ. Caries Res 9:98–109
Sarkar NK (2000) Internal corrosion in dental composite wear: its significance and simulation. J Biomed Mater Res B Appl Biomater 53:371–380
Beech DR, Bandyopadhyai S (1983) A new laboratory method for evaluating the relative solubility and erosion of dental cements. J Oral Rehabil 10:57–63
Ferracane JL (2006) Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater 22:211–222
Göpferich A (1996) Mechanisms of polymer degradation and erosion. Biomaterials 17:103–114
Flemming GJP, Awan M, Cooper PR, Sloan AJ (2008) The potential of a resin-composite to be cured to a 4 mm depth. Dent Mater 24:522–529
Sideridou I, Achilias DS, Spyroudi C, Karabela M (2004) Water sorption characteristics of light-cured dental resins and composites based on Bis-EMA/PCDM. Biomaterials 25:367–376
Acknowledgements
This study was supported by a grant (E-26/171.432/2004) from Rio de Janeiro Research Foundation (FAPERJ). The authors would like to thank 3M ESPE for supplying the Z350 and P60 dental composites; the Institute of Macromolecules (IMA) of the Federal University of Rio de Janeiro (UFRJ)—for performing the degree of conversion measurements, and the Electronic Microscopy Laboratory/PEMM of UFRJ—for performing the SEM analysis.
Conflict interest declaration
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, E.M., Gonçalves, L., Guimarães, J.G.A. et al. The diffusion kinetics of a nanofilled and a midifilled resin composite immersed in distilled water, artificial saliva, and lactic acid. Clin Oral Invest 15, 393–401 (2011). https://doi.org/10.1007/s00784-010-0392-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-010-0392-z