Abstract
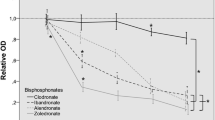
Bisphosphonate-associated osteonecrosis of the jaws (BP-ONJ) is one of the main side effects in patients treated with bisphosphonates for metastasis to the bone or osteoporosis. BP-ONJ usually occurs in patients treated with highly potent nitrogen-containing bisphosphonates. The exact mechanism of action and etiopathology is still unknown. In addition to inhibition of bone remodelling, an anti-angiogenetic effect has become the focus of research. The aim of these study was to investigate the effect of different bisphosphonates on human umbilicord vein endothelial cells (HUVEC) and endothelial progenitor cells (EPC), which play an important role in angiogenesis. Using varying concentrations, the impact of one non-nitrogen-containing bisphosphonate (clodronate) and three nitrogen-containing bisphosphonates (ibandronate, pamidronate and zoledronate) on HUVEC and EPC was analysed. The biologic behaviour of HUVEC after incubation with different bisphosphonates was measured in a Boyden migration assay as well as in a 3D angiogenesis assay. The number of apoptotic cells was measured by Tunnel assay. To underline the importance of neoangiogenesis in the context of BP-ONJ, we measured the EPC number after incubation with different bisphosphonates in vitro. HUVEC and EPC were significantly influenced by bisphosphonates at different concentrations compared with the non-treated control groups. The nitrogen-containing bisphosphonates pamidronate and zoledronate had the greatest impact on the cells, whereas clodronate followed by ibandronate was less distinct on cell function. These results underline the hypothesis that inhibited angiogenesis induced by bisphosphonates might be of relevance in the development and maintenance of BP-ONJ. The increased impact by highly potent bisphosphonates on HUVEC and EPC may explain the high prevalence of BP-ONJ in patients undergoing this treatment.





Similar content being viewed by others
References
Diel IJ, Bergner R, Grotz KA (2007) Adverse effects of bisphosphonates: current issues. J Support Oncol 5(10):475–482
Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of jaws: a growing epidemic. J Oral Maxillofac Surg 61(9):1115–1117
Walter C, Grotz KA, Kunkel M et al (2007) Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer 15(2):197–202
Ruggiero SL, Dodson TB, Assael LA et al (2009) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 67(5 Suppl):2–12
Hoff AO, Toth BB, Altundag K et al (2008) Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 23(6):826–836
Boonyapakorn T, Schirmer I, Reichart PA et al (2008) Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol 44(9):857–869
Walter C, Al-Nawas B, Grotz KA et al (2008) Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur Urol 54(5):1066–1072
Abu-Id MH, Warnke PH, Gottschalk J et al (2008) “Bis-phossy jaws”—high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg 36(2):95–103
Lam DK, Sandor GK, Holmes HI et al (2007) A review of bisphosphonate-associated osteonecrosis of the jaws and its management. J Can Dent Assoc 73(5):417–422
Landesberg R, Cozin M, Cremers S et al (2008) Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg 66(5):839–847
Simon MJ, Niehoff P, Kimmig B et al. (2009) Expression profile and synthesis of different collagen types I, II, III, and V of human gingival fibroblasts, osteoblasts, and SaOS-2 cells after bisphosphonate treatment. Clin Oral Investig. In press
Gerber HP, Ferrara N (2000) Angiogenesis and bone growth. Trends Cardiovasc Med 10(5):223–228
Asahara T, Masuda H, Takahashi T et al (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85(3):221–228
Asahara T, Murohara T, Sullivan A et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275(5302):964–967
Ziebart T, Yoon CH, Trepels T et al (2008) Sustained persistence of transplanted proangiogenic cells contributes to neovascularization and cardiac function after ischemia. Circ Res 103(11):1327–1334
Urbich C, Heeschen C, Aicher A et al (2005) Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med 11(2):206–213
Allegra A, Oteri G, Nastro E et al (2007) Patients with bisphosphonates-associated osteonecrosis of the jaw have reduced circulating endothelial cells. Hematol Oncol 25(4):164–169
Santini D, Vincenzi B, Avvisati G et al (2002) Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin Cancer Res 8(5):1080–1084
Hasmim M, Bieler G, Ruegg C (2007) Zoledronate inhibits endothelial cell adhesion, migration and survival through the suppression of multiple, prenylation-dependent signaling pathways. J Thromb Haemost 5(1):166–173
Mashiba T, Hirano T, Turner CH et al (2000) Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 15(4):613–620
Allen MR, Burr DB (2009) The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg 67(5 Suppl):61–70
Conway EM, Collen D, Carmeliet P (2001) Molecular mechanisms of blood vessel growth. Cardiovasc Res 49(3):507–521
Aicher A, Kollet O, Heeschen C et al (2008) The Wnt antagonist Dickkopf-1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ Res 103(8):796–803
Soltau J, Zirrgiebel U, Esser N et al (2008) Antitumoral and antiangiogenic efficacy of bisphosphonates in vitro and in a murine RENCA model. Anticancer Res 28(2A):933–941
Santini D, Vespasiani Gentilucci U, Vincenzi B et al (2003) The antineoplastic role of bisphosphonates: from basic research to clinical evidence. Ann Oncol 14(10):1468–1476
Wood J, Bonjean K, Ruetz S et al (2002) Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther 302(3):1055–1061
Bezzi M, Hasmim M, Bieler G et al (2003) Zoledronate sensitizes endothelial cells to tumor necrosis factor-induced programmed cell death: evidence for the suppression of sustained activation of focal adhesion kinase and protein kinase B/Akt. J Biol Chem 278(44):43603–43614
Fournier P, Boissier S, Filleur S et al (2002) Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 62(22):6538–6544
Okamoto T, Yamagishi S, Inagaki Y et al (2002) Incadronate disodium inhibits advanced glycation end products-induced angiogenesis in vitro. Biochem Biophys Res Commun 297(2):419–424
Yamada J, Tsuno NH, Kitayama J et al (2009) Anti-angiogenic property of zoledronic acid by inhibition of endothelial progenitor cell differentiation. J Surg Res 151(1):115–120
Frith JC, Monkkonen J, Blackburn GM et al (1997) Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res 12(9):1358–1367
Nogawa M, Yuasa T, Kimura S et al (2005) Zoledronic acid mediates Ras-independent growth inhibition of prostate cancer cells. Oncol Res 15(1):1–9
Bifulco M (2005) Role of the isoprenoid pathway in ras transforming activity, cytoskeleton organization, cell proliferation and apoptosis. Life Sci 77(14):1740–1749
Crick DC, Andres DA, Waechter CJ (1997) Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem Biophys Res Commun 237(3):483–487
Conflict of interest statement
Professor Dr. W. Wagner, PD Dr. B. Al-Nawas and Dr. Ch. Walter gave speeches for Roche. Dr. Ch. Walter received a research fund for another project by Novartis.
Role of the funding source
This study was not funded.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ziebart, T., Pabst, A., Klein, M.O. et al. Bisphosphonates: restrictions for vasculogenesis and angiogenesis: inhibition of cell function of endothelial progenitor cells and mature endothelial cells in vitro. Clin Oral Invest 15, 105–111 (2011). https://doi.org/10.1007/s00784-009-0365-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-009-0365-2