Abstract
The aim of the present study was to investigate bone formation to recombinant human bone morphogenetic protein-2 (rhBMP-2)-biocoated and rhBMP-2-nonbiocoated titanium implants after implantation in dogs. Implantation of sand-blasted and acid-etched (C), chromosulfuric acid surface-enhanced (CSA), and rhBMP-2-biocoated CSA [BMP-A: noncovalently immobilized rhBMP-2 (596 ng/cm2), BMP-B: covalently immobilized rhBMP-2 (819 ng/cm2)] implants was performed in both the mandible and tibia of dogs. After 4 weeks of healing, the percentage of direct bone to implant contact (BIC) and the induced bone density (BD) at a distance of less than and greater than 1 mm adjacent to each implant was assessed. Histomorphometric analysis of implants inserted in the mandible and tibia revealed that BIC values appeared to be highest in the BMP-B group, followed by BMP-A, CSA, and C. BD as measured at a distance of <1 mm revealed obvious differences between groups: BMP-B>BMP-A>CSA>C. However, no differences between groups were observed at a distance of >1 mm. Within the limits of the present study, it may be concluded that rhBMP-2 immobilized by covalent and noncovalent methods on CSA-treated implant surfaces seemed to be stable and promoted direct bone apposition in a concentration-dependant manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adhesion of plasma proteins on the surface of titanium implants has been reported to play an essential role in the process of osseointegration [2, 4, 8, 9]. Each surface of a material is characterized by a unique composition of adsorbed proteins, which influences the type of cells that may adhere. Subsequently, the specific pattern of adsorbed proteins determines the type of tissue that will develop at the interface between the implanted material and the host [32, 41, 42]. In recent years, several modifications of specific surface properties such as structure, chemistry, surface charge, and wettability have been investigated to improve osseointegration of titanium implants [1]. Additionally, several growth factors and cytokines have also been suggested to stimulate a deposition of cells with the capacity of regenerating the desired tissue [27, 39, 46]. In case of endosseous titanium implants, an enhanced proliferation and differentiation of undifferentiated mesenchymal cells, osteoprogenitor cells, and preosteoblasts into osteoblasts may improve bone response and subsequently osseointegration [5]. One particular growth factor, bone morphogenetic protein (BMP), has shown considerable potential to stimulate bone formation both in extraskeletal sites [47, 48] and in defect models in different species [38, 50]. BMPs originate from the transforming growth factor-β family, including at least 18 proteins [29]. BMP-2, which has been described as an anthelix structure, seems to possess the highest osteoinductive potential among the BMPs [25]. In recent years, the regenerative potential of recombinant human BMP-2 (rhBMP-2) has been demonstrated in various experimental animal studies, including sinus floor augmentation, alveolar ridge preservation, bone augmentation procedures, and periodontal repair [3, 11–15, 31, 33, 49]. Most recently, the effects of rhBMP-2 on the osseointegration of titanium implants have also been investigated in experimental animal studies [14, 26, 33–37, 45]. Experimental titanium plasma-sprayed hollow cylinder implants were filled with a solution of rhBMP-2 soaked on an absorbable type-I collagen sponge before insertion. The histomorphometric analysis revealed a significantly increased bone regeneration in rhBMP-2-treated defects compared to controls. Furthermore, the level of osseointegration, as measured by direct bone-implant contact, was significantly higher for the rhBMP-2 implants compared to controls [34]. Recently, biologically active rhBMP-2 has also been covalently immobilized on metal surfaces [16, 19–21, 44]. Osseointegration of rhBMP-2-biocoated plasma spray-coated titanium–alloy cylinders, as evaluated histomorphometrically 4 weeks after implantation in the distal femur condylus in a gap healing model in sheeps, was predominantly characterized by circumferential bone formation and integration with minimal residual gaps. In contrast, control specimens generally exhibited a wide gap surrounding the implant cylinder [18]. The rationale for BMP immobilization was to avoid ectopic bone formation by a limited and targeted release of rhBMP-2 from the implant surface. Recently, treatment of titanium with chromosulfuric acid (CSA) has been reported to result in ultrahydrophilic bioadhesive surfaces, which in turn improves biocoating with rhBMP-2 [19]. However, there are currently no histological data evaluating osseointegration of CSA-modified and rhBMP-2-biocoated titanium implants.
Therefore, the aim of the present study was to investigate histomorphometrically bone formation to CSA-enhanced and rhBMP-2-biocoated titanium implants after implantation in the mandibula and tibia of dogs in comparison to control titanium implants.
Materials and methods
Animals
Two 3-year-old male mongrel dogs (approximate weight 25 kg) were used in the study. Both animals exhibited a fully erupted, healthy, permanent dentition. During the experiment, the dogs were fed ad libitum with soft-food diet and water. Animal selection, management, and surgery protocol were approved by the Animal Care and Use Committee of Belgrade University (ref. no. 2179). The experimental segment of the study started after an adaptation period of 4 weeks.
Study design
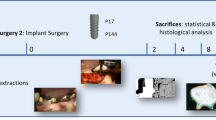
The study was performed in two surgical phases. In the first phase, extraction of the mandibular second, third, and fourth premolar and first molar (P2–M1) was performed bilaterally. After 4 months of healing, surgical implantation of rhBMP-2-biocoated and rhBMP-2-noncoated screw-typed implants was performed in a submerged healing procedure during the second phase. Throughout the study period, oral hygiene procedures were performed three times a week including tooth and implant brushing. Radiographs were obtained before and immediately after tooth extraction as well as immediately after implant installation. Both animals were killed after a healing period of 4 weeks.
Implant preparation
RhBMP-2 was prepared as previously described [21]. The biological activity of soluble rhBMP-2 was assessed with MC3T3-E1 cells by the induction of the de novo synthesis of alkaline phosphatase (AP) [43]. The half-activation constants (K 0.5) were in the range of 20–75 nM [18]. Twenty-four screw-type implants (Camlog Screw Line, Wimsheim, Germany) were manufactured from commercially pure titanium. The core diameter of the implants was 3.3 mm and the total length was 11 mm. A total of six (n=6) implants were sand-blasted and acid-etched according to a standardized procedure (Promote, Altatec, Wimsheim, Germany) (C), while a total of 18 (n=18) implants were surface-enhanced by a novel procedure with CSA [21]. The treatment of metals with CSA (CSA–Ti–alloy) [21] leads to ultrahydrophilic (contact angles 0–10°, no hysteresis) bioadhesive surfaces [17]. A total of 12 (n=12) surface-enhanced implants were divided into two subgroups (A and B) and biocoated with rhBMP-2 [BMP-A: noncovalently immobilized rhBMP-2 (596 ng/cm2), BMP-B: covalently immobilized rhBMP-2 (819 ng/cm2)] [40]. RhBMP-2 was immobilized by covalent and noncovalent methods on these CSA-treated surfaces [18, 21, 40]. In brief, the implants were assigned to the following test and control groups: BMP-A (n=6), BMP-B (n=6), CSA (n=6), and C (n=6).
To control the produced surface, the following “sibling method” was employed: Parallel to the preparation of the above dental implants for in vivo experiments, miniplates (10×5×1 mm) with identical Promote surfaces were surface-enhanced with CSA and coated with 125I-rhBMP-2 under identical conditions as the dental implants. In this way, the corresponding contact angles, the amount of immobilized rhBMP-2, and the in vitro biological activity [6] could be tested before the implants were placed into the animals. Only those dental implants were released for implantation, whose sibling miniplates reached the standard as mentioned above and whose surfaces showed an intense in vitro bioactivity by fluorescence microscopy [6].
Surgical procedure for both phases
The dogs were anesthetized with 1 mg/kg sodium pentobarbital. To maintain hydration, both animals received a constant rate infusion of lactated Ringer’s solution while being anesthetized. Prophylactic antibiotics were administrated intraoperatively with a combination of 20,000 IU penicillin and 1.0 g streptomycin/10 kg body weight. In the first surgery, P2–M1 were carefully removed after reflection of full thickness mucoperiosteal flaps and tooth separation. After wound closure by means of mattress sutures, the sites were allowed to heal for 4 months. In the second surgery, the test and control implants were randomly allocated to both sides of the mandible (left and right sides, one implant each) and one implant each to either the posterior left or right tibia. In brief, bilateral vestibular incisions were made, and full thickness mucoperiosteal flaps were elevated to expose the respective sites for implant placement in the mandible. Surgical implant sites were prepared bilaterally, at a distance of 10 mm apart, according to the protocol suggested by the manufacturer. All implants in the mandible were sealed with cover screws (Camlog, Wimsheim, Germany). After irrigation, mucoperiosteal flaps were repositioned, and primary wound closure was achieved with consecutive polyglycolic acid 5.0 Polyester sutures (Resorba, Nürnberg, Germany). An area of approximately 15 cm in length and 4 cm in width was depilated on the respective side of the tibia using an electric shaver and a razor blade. After disinfection with polyvidone iodine (Betaisodona, Mundipharma, Limburg/Lahn, Germany), a skin incision was made and a flap was elevated to expose the respective sites of the tibia for implant placement. On the inner-posterior side of the tibias, implants were inserted at a distance of 30 mm apart. All titanium implants were inserted with good primary stability according to a low-trauma surgical technique under copious irrigation with sterile 0.9% physiological saline. After implant placement, the periosteum and fascia were sutured using 3.0 Polyester sutures (Resorba, Nürnberg, Germany).
Animal killing and retrieval of specimens
The animals were killed (overdose of sodium pentobarbital, 200 mg/kg i.v.) after 4 weeks. The jaws as well as the posterior tibias were dissected, and blocks containing the experimental specimens were obtained. Block sections of the anterior tibia served as additional control (UC). All specimens were fixed in 10% neutral buffered formalin solution for 4–7 days. The specimens were dehydrated using ascending grades of alcohol and xylol, and infiltrated and embedded in methylmethacrylate (MMA, Technovit 7200, Heraeus Kulzer, Wehrheim, Germany) for nondecalcified sectioning. After 18 to 24 h, the specimens were completely polymerized. Each implant site was cut in the mesiodistal direction and along with the long axis of the implant using a diamond wire saw (Exakt, Apparatebau, Norderstedt, Germany), resulting in four sections of approximately 500 μm in thickness [7]. Subsequently, all specimens were glued with acrylic cement (Technovit 7210 VLC, Heraeus Kulzer, Wehrheim, Germany) to opaque Plexiglas and ground to a final thickness of approximately 40 μm. All sections were stained with toluidine blue.
Histological and histomorphometric analysis
Histomorphometrical analyses as well as microscopic observations were performed by one experienced investigator masked to the specific experimental conditions. For histomorphometrical measurements, images were obtained using a light microscope (BX50, Olympus, Hamburg, Germany) at a magnification of 100×, associated with a video camera (SIS Color View3, Soft imaging System GmbH, Münster, Germany). Digital images were evaluated using a software program (SIS analySIS Auto Software 3.2, Soft imaging System GmbH, Münster, Germany). The percentage of direct contact between mineralized bone and the titanium surface (bone to implant contact—BIC) was measured at every thread on both sides of the implant [23]. BIC was subdivided into crestal and apical values (upper and lower halves of the intraosseous implant length, respectively). Furthermore, in the tibias, induced bone density (BD) adjacent to the titanium surfaces was measured at distances of <1 and 1 mm by assessing the ratio of mineralized bone vs bone marrow within the respective areas [28].
Results
Clinical observations
The postoperative healing was uneventful in both dogs. No complications such as allergic reactions, abscesses, or infections were observed throughout the study period.
Histological and histomorphometric analysis
BIC and BD values for each group and respective anatomical sites (mandible and tibia) are presented in Fig. 1a–c. In particular, all test and control implants inserted in the mandible generally exhibited new bone formation in direct contact with the implant interface. The formation of organized trabeculas of woven bone, recognized by osteon formation, could be observed in all groups. However, new bone formation in direct contact with the implant interface appeared to be higher in the BMP groups (Fig. 2a–d). In particular, BIC values appeared to be highest in the BMP-B group, followed by BMP-A, CSA, and C groups. No differences in BIC with respect to crestal and apical values were observed within or between groups (Fig. 1a).
Boxplots with outliners for the medians and Q1–Q3 quartiles of BIC and BD (%) in different groups after 4 weeks of healing with respective values in the crestal and apical portion of the implant (BIC) and at a distance of less than and greater than 1 mm adjacent to the implant surface (BD). Lines below and above box plots min, max. control c. a BIC mandibula. b BIC tibia. c BD tibia
In comparison to the organized trabeculas of woven bone noted in the mandible, bone formation in the tibia seemed to be of a cancellous type, mainly characterized by tiny trabeculas (Fig. 3a–d). BIC values appeared to be highest in the BMP-B group, followed by BMP-A, CSA, and C groups. Again, with respect to crestal and apical BIC values, no differences were observed within groups (Fig. 1b). Histomorphometric analysis of BD in the tibia at a distance of <1 mm revealed obvious differences between groups. In particular, highest values were noted for both BMP groups (BMP-A=BMP-B). This was followed by CSA, also exhibiting higher BD values than C. No differences between groups were found at a distance of >1 mm (Fig. 2c).
Discussion
The present histological study was designed to evaluate bone formation and direct bone apposition to rhBMP-2-biocoated, CSA surface-enhanced, and C titanium implants after implantation in the mandible and tibia of dogs. In particular, rhBMP-2 was immobilized by covalent and noncovalent methods on CSA surface-enhanced titanium implants [18, 21, 40]. Within its limits, histomorphometrical analysis of implants inserted in both mandible and tibia after 4 weeks revealed that BIC values appeared to be highest in the BMP groups, followed by CSA and C groups. Furthermore, it was observed that BD as measured at a distance of <1 mm in the tibia was obviously highest in the BMP-B group, followed by BMP-A, CSA, and C groups. However, no differences between groups were observed at a distance of >1 mm. In this context, it is important to realize that the present pilot study does not have the statistical power to rule out the possibility of a difference between groups. Further experimental studies of higher power are needed to support equivalence or superiority [10]. On the other hand, it needs also to be pointed out that these are the first histological data evaluating bone formation and apposition on rhBMP-2-biocoated and CSA surface-enhanced titanium implants in the mandible and tibia. However, the present findings corroborate, to a certain extent, previous results observed in an ectopic bone formation model [40]. Electropolished titanium miniplates were surface-enhanced by CSA and coated with a total amount of 150–200 ng rhBMP-2. Periosteal flaps were prepared from the anterior surface of the tibias of adult rabbits and wrapped around the titanium specimens. Additionally, some titanium miniplates were inserted to which nonimmobilized soluble rhBMP-2 was added. After 28 days of healing, noncoated specimens revealed bone formation in 2/12 implants, rhBMP-2-coated implants in 6/8, and implants with free rhBMP-2 in 8/8 cases. However, in the case of rhBMP-2-coated implants, the induced bone had direct contact to the implant in all cases. In contrast, titanium miniplates inserted with free administered rhBMP-2 revealed direct BIC in just six cases, whereas in two cases, the titanium surface was separated by a fibrous capsule [40]. The finding that rhBMP-2 may promote periimplant bone regeneration and osseointegration of titanium implants is in accordance with the previous studies [14, 26, 33–37, 45]. In all of these studies, however, rhBMP-2 was admixed with a carrier (i.e., collagen, calcium-phosphate cement carrier), acting as a slow delivery system, for instillation with the implant due to a rapid diffusion of BMP after implantation in vivo. In particular, Sigurdsson et al. [33] evaluated rhBMP-2- (2×0.43 mg/ml in a type-I bovine collagen carrier) induced bone regeneration and osseointegration in a supraalveolar periimplant defect model in dogs. At 16 weeks after healing, bone regeneration (height) was significantly larger for rhBMP-2 than control defects. However, the large amount of BMP-induced bone was poorly adapted to the implant surface. In contrast, the smaller amount of new bone in the control group seemed to be well adapted [33]. In contrast, Wikesjö et al. [45] reported similar BIC values 16 weeks after implantation of rhBMP-2 (0.4 and 0.75 mg/ml in a calcium-phosphate cement carrier) or carrier alone subsequent to a vertical alveolar ridge augmentation procedure and simultaneous implant installation in dogs. Furthermore, Howell et al. [14] applied rhBMP-2 using a collagen sponge carrier to stimulate bone formation in artificially created defects around endosseous implants in the canine mandible. Nonresorbable expanded tetrafluoroethylene (e-PTFE) membranes served as controls. Histological analysis revealed that the addition of rhBMP-2 resulted in a significantly greater amount of new bone area and BIC after 4 and 12 weeks of healing than e-PTFE. Although membrane-treated sites were reported to have less new bone formation after 4 weeks of healing, this difference seemed to be equalized after 12 weeks [14]. Similar results were also reported by Sykaras et al. [34]. RhBMP-2-induced bone regeneration and osseointegration was evaluated in mandibular bony defects created within the hollow chamber of endosseous dental implants in dogs. Before insertion, hollow chambers were filled with 20 μg of rhBMP-2 soaked on an absorbable type-I collagen sponge (0.4 mg/ml). Histological observation revealed statistically significant higher BIC values in the rhBMP-2 group at 8 and 12 weeks after implantation [34]. There might be several explanations for the discrepancies noted in these studies. First of all, it must be emphasized that little information is available on the interaction between rhBMP-2 and the individual carriers. Furthermore, the high concentrations of rhBMP-2 used in these studies (in the milligram per milliliter range) strongly indicate that an optimal method is still lacking. In this context, it is important to point out that BMP-2 has the potency to induce or modulate apoptosis [24], and that in vivo application of high doses of BMP-2 may inhibit bone formation [22, 30]. Furthermore, the results of a recent cell culture study have shown that the dose-dependent effect of rhBMP-2 on AP induction in MC3T3-E1 cells plateaus out into a maximal response at 300–1,000 nM BMP-2 (i.e., 8–25 μg/ml) [43]. Indeed, the results of the present study have shown that BIC and BD values seemed to be ameliorated after application of rhBMP-2 at far lower concentrations. Furthermore, it must also be noted that the effects of rhBMP-2 were limited to a range of 1 mm, outlining that both covalent and noncovalent methods of immobilization seemed to be stable. This finding may also be supported by the observation that no differences with respect to BIC values were observed in crestal and apical areas of the implant surface in all groups. In accordance, it might be hypothesized that from a clinical point of view, both methods of immobilization are suitable to avoid ectopic bone formation due to a limited and targeted release of rhBMP-2 from the implant surface. However, further studies are needed to clarify this issue.
Within the limits of the present study, it may be concluded that rhBMP-2 immobilized by covalent and noncovalent methods on CSA-treated implant surfaces seemed to be stable and promoted direct bone apposition in a concentration-dependent manner.
References
Albrektsson T, Brånemark PI, Hansson HA, Lindstrom J (1981) Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand 52:155–170
Baier RE, Dutton RC (1969) Initial events in interactions of blood with a foreign surface. J Biomed Mater Res 3:191–206
Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, Alder M, Nummikoski P (1997) A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent 17:11–25
Brash JL, ten Hove P (1984) Effect of plasma dilution on adsorption of fibrinogen to solid surfaces. Thromb Haemost 51:326–330
Chappard D, Aguado E, Hure G, Grizon F, Basle MF (1999) The early remodeling phases around titanium implants: a histomorphometric assessment of bone quality in a 3- and 6-month study in sheep. Int J Oral Maxillofac Implants 14:189–196
Chatzinikolaidou M, Zumbrink T, Jennissen HP (2003) Stability of surface-enhanced ultrahydrophilic metals as a basis for bioactive rhBMP-2 surfaces. Materialwiss Werkstofftech 34:1106–1112
Donath K (1985) The diagnostic value of the new method for the study of undecalcified bones and teeth with attached soft tissue (Säge–Schliff (sawing and grinding) technique). Pathol Res Pract 179:631–633
Eriksson C, Lausmaa J, Nygren H (2001) Interactions between human whole blood and modified TiO2-surfaces: influence of surface topography and oxide thickness on leukocyte adhesion and activation. Biomaterials 22:1987–1996
Eriksson C, Nygren H (2001) Polymorphonuclear leukocytes in coagulating whole blood recognize hydrophilic and hydrophobic titanium surfaces by different adhesion receptors and show different patterns of receptor expression. J Lab Clin Med 137:296–302
Gunsolley JC, Elswick RK, Davenport JM (1998) Equivalence and superiority testing in regeneration clinical trials. J Periodontol 69:521–527
Hanisch O, Tatakis DN, Boskovic MM, Rohrer MD, Wikesjo UM (1997) Bone formation and reosseointegration in peri-implantitis defects following surgical implantation of rhBMP-2. Int J Oral Maxillofac Implants 12:604–610
Hanisch O, Tatakis DN, Rohrer MD, Wohrle PS, Wozney JM, Wikesjo UM (1997) Bone formation and osseointegration stimulated by rhBMP-2 following subantral augmentation procedures in nonhuman primates. Int J Oral Maxillofac Implants 12:785–792
Hollinger JO, Schmitt JM, Buck DC, Shannon R, Joh SP, Zegzula HD, Wozney J (1998) Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J Biomed Mater Res 43:356–364
Howell TH, Fiorellini J, Jones A, Alder M, Nummikoski P, Lazaro M, Lilly L, Cochran D (1997) A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int J Periodontics Restorative Dent 17:124–139
Inoda H, Yamamoto G, Hattori T (2004) Histological investigation of osteoinductive properties of rh-BMP2 in a rat calvarial bone defect model. J Craniomaxillofac Surg 32:365–369
Jennissen HP (1999) Method for immobilizing mediator molecules on inorganic and metal implant materials. PCT Patent WO9926674A2, European Patent Office, Munich, Germany
Jennissen HP (2001) Ultra-hydrophile metallische Biomaterialien. Biomaterialien 2:45–53
Jennissen HP, Chatzinikolaidou M, Rumpf HM (2000) Modification of metal surfaces and biocoating of implants with bone-morphogenetic protein 2 (BMP-2). DVM Bericht 313:127–140
Jennissen HP, Zumbrink T (1999) Immobilization of BMP-2 on titanium surfaces. Biol Chem 380:161
Jennissen HP, Zumbrink T (1999) Protein immobilization on metal implant surfaces with potential for biocoating with BMPs. FASEB J 13:427 (Abstract)
Jennissen HP, Zumbrink T, Chatzinikolaidou M, Steppuhn J (1999) Biocoating of implants with mediator molecules: surface enhancement of metals by treatment with chromosulfuric acid. Materialwiss Werkstofftech 30:838–845
Jeppsson C, Aspenberg P (1996) BMP-2 can inhibit bone healing. Bone-chamber study in rabbits. Acta Orthop Scand 67:589–592
Johansson CB, Albrektsson T (1991) A removal torque and histomorphometric study of commercially pure niobium and titanium implants in rabbit bone. Clin Oral Implants Res 2:24–29
Kawamura C, Kizaki M, Yamato K, Uchida H, Fukuchi Y, Hattori Y, Koseki T, Nishihara T, Ikeda Y (2000) Bone morphogenetic protein-2 induces apoptosis in human myeloma cells with modulation of STAT3. Blood 96:2005–2011
Laub MT, Seul E, Schmachtenberg E, Jennissen HP (2001) Molecular modelling of bone-morphogenetic protein 2 (BMP-2) by 3D-rapid prototyping. Materialwiss Werkstofftech 32:926–930
Matin K, Senpuku H, Hanada N, Ozawa H, Ejiri S (2003) Bone regeneration by recombinant human bone morphogenetic protein-2 around immediate implants: a pilot study in rats. Int J Oral Maxillofac Implants 18:211–217
Mohan S, Baylink DJ (1991) Bone growth factors. Clin Orthop Relat Res 263:30–48
Nociti FH, Jr, Cesar NJ, Carvalho MD, Sallum EA (2002) Bone density around titanium implants may be influenced by intermittent cigarette smoke inhalation: a histometric study in rats. Int J Oral Maxillofac Implants 17:347–352
Reddi AH (1995) Bone morphogenetic proteins, bone marrow stromal cells, and mesenchymal stem cells. Maureen Owen revisited. Clin Orthop Relat Res 313:115–119
Ritter SJ, Davies PJ (1998) Identification of a transforming growth factor-beta1/bone morphogenetic protein 4 (TGF-beta1/BMP4) response element within the mouse tissue transglutaminase gene promoter. J Biol Chem 273:12798–12806
Sailer HF, Kolb E (1994) Application of purified bone morphogenetic protein (BMP) preparations in cranio-maxillo-facial surgery. Reconstruction in craniofacial malformations and post-traumatic or operative defects of the skull with lyophilized cartilage and BMP. J Craniomaxillofac Surg 22:191–199
Schmaier AH, Silver L, Adams AL, Fischer GC, Munoz PC, Vroman L, Colman RW (1984) The effect of high molecular weight kininogen on surface-adsorbed fibrinogen. Thromb Res 33:51–67
Sigurdsson TJ, Nygaard L, Tatakis DN, Fu E, Turek TJ, Jin L, Wozney JM, Wikesjo UM (1996) Periodontal repair in dogs: evaluation of rhBMP-2 carriers. Int J Periodontics Restorative Dent 16:524–537
Sykaras N, Triplett RG, Nunn ME, Iacopino AM, Opperman LA (2001) Effect of recombinant human bone morphogenetic protein-2 on bone regeneration and osseointegration of dental implants. Clin Oral Implants Res 12:339–349
Sykaras N, Iacopino AM, Triplett RG, Marker VA (2004) Effect of recombinant human bone morphogenetic protein-2 on the osseointegration of dental implants: a biomechanics study. Clin Oral Investig 8:196–205
Sykaras N, Woody RD, Lacopino AM, Triplett RG, Nunn ME (2004) Osseointegration of dental implants complexed with rhBMP-2: a comparative histomorphometric and radiographic evaluation. Int J Oral Maxillofac Implants 19:667–678
Tatakis DN, Koh A, Jin L, Wozney JM, Rohrer MD, Wikesjo UM (2002) Peri-implant bone regeneration using recombinant human bone morphogenetic protein-2 in a canine model: a dose-response study. J Periodontal Res 37:93–100
Teixeira JO, Urist MR (1998) Bone morphogenetic protein induced repair of compartmentalized segmental diaphyseal defects. Arch Orthop Trauma Surg 117:27–34
Urist MR, DeLange RJ, Finerman GA (1983) Bone cell differentiation and growth factors. Science 220:680–686
Voggenreiter GK, Hartl M, Assenmacher S, Chatzinikolaidou M, Jennissen HP, Rumpf HM (2001) Assessment of the biological activity of chemically immobilized rhBMP-2 on titanium surfaces in vivo. Materialwiss Werkstofftech 32:942–948
Walivaara B, Aronsson BO, Rodahl M, Lausmaa J, Tengvall P (1994) Titanium with different oxides: in vitro studies of protein adsorption and contact activation. Biomaterials 15:827–834
Walivaara B, Askendal A, Elwing H, Lundstrom I, Tengvall P (1992) Antisera binding onto metals immersed in human plasma in vitro. J Biomed Mater Res 26:1205–1216
Wiemann M, Rumpf HM, Bingmann D, Jennissen HP (2001) The binding of rhBMP-2 to the receptors of MC3T3-E1 cells and the question of cooperativity. Materialwiss Werkstofftech 32:931–936
Wiemann M, Zumbrink T, Jennissen HP, Brauer H, Fischer A, Bingmann D (1999) BMP-2 stimulation of osteoblast-like MC3T3-E1 cells grown on modified metal surfaces. Pflügers Arch 437:R106
Wikesjö UM, Sorensen RG, Kinoshita A, Wozney JM (2002) RhBMP-2/alphaBSM induces significant vertical alveolar ridge augmentation and dental implant osseointegration. Clin Implant Dent Relat Res 4:174–182
Wozney JM, Rosen V, Byrne M, Celeste AJ, Moutsatsos I, Wang EA (1990) Growth factors influencing bone development. J Cell Sci Suppl 13:149–156
Yamazaki Y, Oida S, Ishihara K, Nakabayashi N (1996) Ectopic induction of cartilage and bone by bovine bone morphogenetic protein using a biodegradable polymeric reservoir. J Biomed Mater Res 30:1–4
Yoshida K, Bessho K, Fujimura K, Kusumoto K, Ogawa Y, Tani Y, Iizuka T (1998) Osteoinduction capability of recombinant human bone morphogenetic protein-2 in intramuscular and subcutaneous sites: an experimental study. J Craniomaxillofac Surg 26:112–115
Zegzula HD, Buck DC, Brekke J, Wozney JM, Hollinger JO (1997) Bone formation with use of rhBMP-2 (recombinant human bone morphogenetic protein-2). J Bone Joint Surg Am 79:1778–1790
Zellin G, Linde A (1997) Treatment of segmental defects in long bones using osteopromotive membranes and recombinant human bone morphogenetic protein-2. An experimental study in rabbits. Scand J Plast Reconstr Surg Hand Surg 31:97–104
Acknowledgements
We highly appreciate the competent laboratory assistance of Dr. Monika Herten, Ms. Brigitte Hartig, Mr. Daniel Ferrari (Department of Oral Surgery, Heinrich Heine University, Düsseldorf, Germany), and Dr. Markus Laub (Institute of Physiology, University of Essen, Essen, Germany). Furthermore, we would like to thank Dr. Zoran Aleksic (Department of Periodontology, University of Belgrade, Belgrade, Serbia and Montenegro) for the help in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00784-006-0069-9
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Becker, J., Kirsch, A., Schwarz, F. et al. Bone apposition to titanium implants biocoated with recombinant human bone morphogenetic protein-2 (rhBMP-2). A pilot study in dogs. Clin Oral Invest 10, 217–224 (2006). https://doi.org/10.1007/s00784-006-0049-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-006-0049-0