Abstract
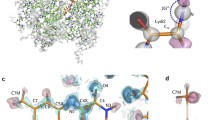
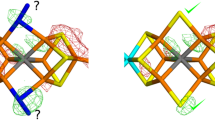
The high-resolution crystal structure of the small iron-sulfur protein rubredoxin (Rd) from the hyperthermophilic archeon Pyrococcus furiosus (Pf) is reported in this paper, together with those of its methionine ([_0M]Pf Rd) and formylmethionine (f[_0M]Pf Rd) variants. These studies were conducted to assess the consequences of the presence or absence of a salt bridge between the amino terminal nitrogen of Ala1 and the side chain of Glu14 to the structure and stability of this rubredoxin. The structure of wild-type Pf Rd was solved to a resolution of 0.95 Å and refined by full-matrix least-squares techniques to a crystallographic agreement factor of 12.8% [F>2σ(F) data, 25 617 reflections], while those of the [_0M]Pf and f[_0M]Pf Rd variants were solved at slightly lower resolutions (1.1 Å, R=11.5%, 17 213 reflections; 1.2 Å, R=13.7%, 12 478 reflections, respectively). The quality of the data was such that about half of the hydrogen atoms of the protein were clearly visible. All three structures were ultimately refined using the program SHELXL-93 with anisotropic atomic displacement parameters for all non-hydrogen protein atoms, and calculated hydrogen positions included but not refined. In this paper we also report thermostability data for all three forms of Pf Rd, and show that they follow the sequence wild-type >[_0M]Pf>formyl[_0M]Pf. Comparison of the three Pf Rd structures in the N-terminal region show that the structures of wild-type Pf Rd and f[_0M]Pf are rather similar, while that of [_0M]Pf Rd shows a number of additional hydrogen bonds involving the extra methionine group. While the salt bridge between the Ala1 amino group and the Glu14 carboxylate is not the primary determinant of the thermostability of Pf Rd, alterations to the amino terminus do have a moderate influence on the thermostability of this protein.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 27 March 1998 / Accepted: 25 June 1998
Rights and permissions
About this article
Cite this article
Bau, R., Rees, D., Kurtz Jr., D. et al. Crystal structure of rubredoxin from Pyrococcus furiosus at 0.95 Å resolution, and the structures of N-terminal methionine and formylmethionine variants of Pf Rd. Contributions of N-terminal interactions to thermostability. JBIC 3, 484–493 (1998). https://doi.org/10.1007/s007750050258
Issue Date:
DOI: https://doi.org/10.1007/s007750050258