Abstract
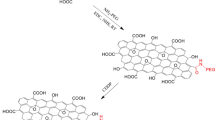
A new multifunctional graphene oxide/Cu (II)-porphyrin MOF nanocomposite (CuG) comprised of Cu-TCPP MOF supported on graphene oxide (GO) nanosheets, has been fabricated by a solvothermal method at low temperature and one-pot process. Cu-TCPP MOF with universal advantages, such as high porosity, nontoxicity, large surface area, and safe biodegradation, combined with GO allows the achievement of an efficient doxorubicin loading (45.7%) and smart pH-responsive release for chemotherapy. More significantly, more than 97% of DOX was released by CuG at pH 5 which was more than that at pH 7.4 (~ 33.5%), while Cu-TCPP MOF displayed DOX release of 68.5% and 49% at pH 5 and 7.4, respectively, illustrating the effect of GO on the smart MOF construction for controllable releasing behavior in vitro. The results of in vitro anticancer experiments demonstrate that the developed nanocarrier exhibited slight or no cytotoxicity on normal cells, while the drug-loaded nanocarrier increased significant cancer cell-killing ability with higher therapeutic efficacy than free DOX, indicating the sustained release behavior of the CuG nanocarrier without any “burst effect”. Moreover, the in vivo experiments demonstrated that the CuG-DOX exhibited significantly higher anticancer efficiency compared with free DOX. High anti-cancer therapeutic efficacy of this nanoscale carrier as an efficient pH sensitive agent, has the potential to enter further biomedical investigations.
Graphic abstract

A new smart multifunctional graphene oxide–Cu (II)-porphyrin MOF nanocomposite (CuG) formed of Cu-TCPP MOF and graphene oxide (GO) has successfully fabricated and demonstrated an efficient pH-responsive drug release behavior in cancer therapy without using any targeting ligand.









Similar content being viewed by others
References
Ke F, Yuan Y-P, Qiu L-G, Shen Y-H, Xie A-J, Zhu J-F, Tian X-Y, Zhang L-D (2011) Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J Mater Chem 21(11):3843–3848
Torad NL, Li Y, Ishihara S, Ariga K, Kamachi Y, Lian H-Y, Hamoudi H, Sakka Y, Chaikittisilp W, Wu KC-W (2014) MOF-derived nanoporous carbon as intracellular drug delivery carriers. Chem Lett 43(5):717–719
Gooneh-Farahani S, Naimi-Jamal MR, Naghib SM (2019) Stimuli-responsive graphene-incorporated multifunctional chitosan for drug delivery applications: a review. Expert Opin Drug Deliv 16(1):79–99
Yang D, Yang G, Gai S, He F, An G, Dai Y, Lv R, Yang P (2015) Au 25 cluster functionalized metal–organic nanostructures for magnetically targeted photodynamic/photothermal therapy triggered by single wavelength 808 nm near-infrared light. Nanoscale 7(46):19568–19578
Bhattacharjee A, Gumma S, Purkait MK (2018) Fe3O4 promoted metal organic framework MIL-100 (Fe) for the controlled release of doxorubicin hydrochloride. Microporous Mesoporous Mater 259:203–210
Xue Y-N, Huang Z-Z, Zhang J-T, Liu M, Zhang M, Huang S-W, Zhuo R-X (2009) Synthesis and self-assembly of amphiphilic poly (acrylic acid-b-DL-lactide) to form micelles for pH-responsive drug delivery. Polymer 50(15):3706–3713
Wang D, Zhou J, Chen R, Shi R, Xia G, Zhou S, Liu Z, Zhang N, Wang H, Guo Z (2016) Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4@ C@ MIL-100 (Fe) nanoparticles. Biomaterials 107:88–101
He C, Lu K, Liu D, Lin W (2014) Nanoscale metal–organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc 136(14):5181–5184
Cunha D, Ben Yahia M, Hall S, Miller SR, Chevreau H, Elkaïm E, Maurin G, Horcajada P, Serre C (2013) Rationale of drug encapsulation and release from biocompatible porous metal–organic frameworks. Chem Mater 25(14):2767–2776
Zhu X, Gu J, Wang Y, Li B, Li Y, Zhao W, Shi J (2014) Inherent anchorages in UiO-66 nanoparticles for efficient capture of alendronate and its mediated release. Chem Commun 50(63):8779–8782
Horcajada P, Serre C, Vallet-Regí M, Sebban M, Taulelle F, Férey G (2006) Metal–organic frameworks as efficient materials for drug delivery. Angew Chem 118(36):6120–6124
Liu W, Pan Y, Xiao W, Xu H, Liu D, Ren F, Peng X, Liu J (2019) Recent developments on zinc (ii) metal–organic framework nanocarriers for physiological pH-responsive drug delivery. MedChemComm 10(12):2038–2051
Zeng J-Y, Wang X-S, Zhang M-K, Li Z-H, Gong D, Pan P, Huang L, Cheng S-X, Cheng H, Zhang X-Z (2017) Universal porphyrinic metal-organic framework coating to various nanostructures for functional integration. ACS Appl Mater Interfaces 9(49):43143–43153
Leng X, Huang H, Wang W, Sai N, You L, Yin X, Ni J (2018) Zirconium-porphyrin PCN-222: pH-responsive controlled anticancer drug Oridonin. Evid-Based Complement Altern Med 2018:1–12
Liu W, Wang YM, Li YH, Cai SJ, Yin XB, He XW, Zhang YK (2017) Fluorescent imaging-guided chemotherapy-and-photodynamic dual therapy with nanoscale porphyrin metal–organic framework. Small 13(17):1603459
Ma Y, Li X, Li A, Yang P, Zhang C, Tang B (2017) H2S-activable MOF nanoparticle photosensitizer for effective photodynamic therapy against cancer with controllable singlet-oxygen release. Angew Chem 129(44):13940–13944
Lv F, Mao L, Liu T (2014) Thermosensitive porphyrin-incorporated hydrogel with four-arm PEG–PCL copolymer: preparation, characterization and fluorescence imaging in vivo. Mater Sci Eng, C 43:221–230
Parida MR, Aly SM, Alarousu E, Mohammed OF (2015) Tunable photophysical processes of porphyrin macrocycles on the surface of ZnO nanoparticles. J Phys Chem C 119(5):2614–2621
Rahimi R, Zargari S, Yousefi A, Berijani MY, Ghaffarinejad A, Morsali A (2015) Visible light photocatalytic disinfection of E. coli with TiO2–graphene nanocomposite sensitized with tetrakis (4-carboxyphenyl) porphyrin. Appl Surf Sci 355:1098–1106
Wei Y, Zhou F, Zhang D, Chen Q, Xing D (2016) A graphene oxide based smart drug delivery system for tumor mitochondria-targeting photodynamic therapy. Nanoscale 8(6):3530–3538
Liang J, Chen B, Hu J, Huang Q, Zhang D, Wan J, Hu Z, Wang B (2019) pH and thermal dual-responsive graphene oxide nanocomplexes for targeted drug delivery and photothermal-chemo/photodynamic synergetic therapy. ACS Appl Bio Mater 2(12):5859–5871
Javanbakht S, Pooresmaeil M, Namazi H (2019) Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohyd Polym 208:294–301
Pumera M (2011) Graphene-based nanomaterials for energy storage. Energy Environ Sci 4(3):668–674
Zhang S, Du Z, Li G (2013) Metal-organic framework-199/graphite oxide hybrid composites coated solid-phase microextraction fibers coupled with gas chromatography for determination of organochlorine pesticides from complicated samples. Talanta 115:32–39
Js G-L, Ortuño-Lizarán I, Fernández-Sanchez L, Alió JL, Ns C, Vega-Estrada A, Silvestre-Albero J (2018) Metal-organic frameworks as drug delivery platforms for ocular therapeutics. ACS Appl Mater Interfaces 11(2):1924–1931
Chowdhuri AR, Singh T, Ghosh SK, Sahu SK (2016) Carbon dots embedded magnetic nanoparticles@ chitosan@ metal organic framework as a nanoprobe for pH sensitive targeted anticancer drug delivery. ACS Appl Mater Interfaces 8(26):16573–16583
Lin W, Hu Q, Jiang K, Yang Y, Yang Y, Cui Y, Qian G (2016) A porphyrin-based metal–organic framework as a pH-responsive drug carrier. J Solid State Chem 237:307–312
Chowdhuri AR, Bhattacharya D, Sahu SK (2016) Magnetic nanoscale metal organic frameworks for potential targeted anticancer drug delivery, imaging and as an MRI contrast agent. Dalton Trans 45(7):2963–2973
Ghahremani F, Shahbazi-Gahrouei D, Kefayat A, Motaghi H, Mehrgardi MA, Javanmard SH (2018) AS1411 aptamer conjugated gold nanoclusters as a targeted radiosensitizer for megavoltage radiation therapy of 4T1 breast cancer cells. RSC Adv 8(8):4249–4258
Kefayat A, Ghahremani F, Motaghi H, Mehrgardi MA (2019) Investigation of different targeting decorations effect on the radiosensitizing efficacy of albumin-stabilized gold nanoparticles for breast cancer radiation therapy. Eur J Pharm Sci 130:225–233
Kefayat A, Ghahremani F, Safavi A, Hajiaghababa A, Moshtaghian J (2019) c-phycocyanin: a natural product with radiosensitizing property for enhancement of colon cancer radiation therapy efficacy through inhibition of COX-2 expression. Sci Rep 9(1):1–13
Kefayat A, Ghahremani F, Safavi A, Hajiaghababa A, Moshtaghian J (2020) Spirulina extract enriched for Braun-type lipoprotein (Immulina®) for inhibition of 4T1 breast tumors’ growth and metastasis. Phytother Res 34(2):368–378
Jani AB, Schreibmann E, Goyal S, Halkar R, Hershatter B, Rossi PJ, Shelton JW, Patel PR, Xu KM, Goodman M (2021) 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial. Lancet 397(10288):1895–1904
Workman P, Aboagye E, Balkwill F, Balmain A, Bruder G, Chaplin D, Double J, Everitt J, Farningham D, Glennie M (2010) Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102(11):1555–1577
Wallace J (2000) Humane endpoints and cancer research. ILAR J 41(2):87–93
Rahimi R, Shariatinia S, Zargari S, Berijani MY, Ghaffarinejad A, Shojaie ZS (2015) Synthesis, characterization, and photocurrent generation of a new nanocomposite based Cu–TCPP MOF and ZnO nanorod. RSC Adv 5(58):46624–46631
Xu G, Yamada T, Otsubo K, Sakaida S, Kitagawa H (2012) Facile “modular assembly” for fast construction of a highly oriented crystalline MOF nanofilm. J Am Chem Soc 134(40):16524–16527
Motoyama S, Makiura R, Sakata O, Kitagawa H (2011) Highly crystalline nanofilm by layering of porphyrin metal−organic framework sheets. J Am Chem Soc 133(15):5640–5643
Hontoria-Lucas C, López-Peinado A, López-González JdD, Rojas-Cervantes M, Martin-Aranda R (1995) Study of oxygen-containing groups in a series of graphite oxides: physical and chemical characterization. Carbon 33(11):1585–1592
Cai D, Song M (2007) Preparation of fully exfoliated graphite oxide nanoplatelets in organic solvents. J Mater Chem 17(35):3678–3680
Rance GA, Marsh DH, Nicholas RJ, Khlobystov AN (2010) UV–vis absorption spectroscopy of carbon nanotubes: relationship between the π-electron plasmon and nanotube diameter. Chem Phys Lett 493(1–3):19–23
Zhou W, Hu B, Liu Z (2009) Metallo-deuteroporphyrin complexes derived from heme: a homogeneous catalyst for cyclohexane oxidation. Appl Catal A 358(2):136–140
Rahimi R, Moghaddas MM, Zargari S (2013) Investigation of the anchoring silane coupling reagent effect in porphyrin sensitized mesoporous V-TiO 2 on the photodegradation efficiency of methyl orange under visible light irradiation. J Sol-Gel Sci Technol 65(3):420–429
Sato T, Mori W, Kato CN, Yanaoka E, Kuribayashi T, Ohtera R, Shiraishi Y (2005) Novel microporous rhodium (II) carboxylate polymer complexes containing metalloporphyrin: syntheses and catalytic performances in hydrogenation of olefins. J Catal 232(1):186–198
Jahan M, Bao Q, Loh KP (2012) Electrocatalytically active graphene–porphyrin MOF composite for oxygen reduction reaction. J Am Chem Soc 134(15):6707–6713
Dresselhaus MS (2012) Fifty years in studying carbon-based materials. Phys Scr 2012(T146):014002
Wang P, Wang J, Wang X, Yu H, Yu J, Lei M, Wang Y (2013) One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl Catal B 132:452–459
Blanton TN, Majumdar D (2013) Characterization of X-ray irradiated graphene oxide coatings using X-ray diffraction, X-ray photoelectron spectroscopy, and atomic force microscopy. Powder Diffr 28(2):68–71
Zhou X, Huang W, Shi J, Zhao Z, Xia Q, Li Y, Wang H, Li Z (2014) A novel MOF/graphene oxide composite GrO@ MIL-101 with high adsorption capacity for acetone. J Mater Chem A 2(13):4722–4730
Karimzadeh Z, Javanbakht S, Namazi H (2019) Carboxymethylcellulose/MOF-5/Graphene oxide bio-nanocomposite as antibacterial drug nanocarrier agent. Bioimpacts 9(1):5
Liu S, Sun L, Xu F, Zhang J, Jiao C, Li F, Li Z, Wang S, Wang Z, Jiang X (2013) Nanosized Cu-MOFs induced by graphene oxide and enhanced gas storage capacity. Energy Environ Sci 6(3):818–823
Murakami H, Nomura T, Nakashima N (2003) Noncovalent porphyrin-functionalized single-walled carbon nanotubes in solution and the formation of porphyrin–nanotube nanocomposites. Chem Phys Lett 378(5–6):481–485
Kanwal U, Bukhari NI, Rana NF, Rehman M, Hussain K, Abbas N, Mehmood A, Raza A (2019) Doxorubicin-loaded quaternary ammonium palmitoyl glycol chitosan polymeric nanoformulation: uptake by cells and organs. Int J Nanomed 14:1
Yang X, Lu Y, Ma Y, Li Y, Du F, Chen Y (2006) Noncovalent nanohybrid of ferrocene with single-walled carbon nanotubes and its enhanced electrochemical property. Chem Phys Lett 420(4–6):416–420
Hu T, Qahtan ASA, Lei L, Lei Z, Zhao D, Nie H (2018) Inhibition of HeLa cell growth by doxorubicin-loaded and tuftsin-conjugated arginate-PEG microparticles. Bioactive Mater 3(1):48–54
OuYang F, Huang B, Li Z, Xiao J, Wang H, Xu H (2008) Chemical functionalization of graphene nanoribbons by carboxyl groups on Stone-Wales defects. J Phys Chem C 112(31):12003–12007
Luan Y, Qi Y, Jin Z, Peng X, Gao H, Wang G (2015) Synthesis of a flower-like Zr-based metal–organic framework and study of its catalytic performance in the Mannich reaction. RSC Adv 5(25):19273–19278
Matvienko T, Sokolova V, Prylutska S, Harahuts Y, Kutsevol N, Kostjukov V, Evstigneev M, Prylutskyy Y, Epple M, Ritter U (2019) In vitro study of the anticancer activity of various doxorubicin-containing dispersions. Bioimpacts 9(1):57
Yang X, Zhang X, Liu Z, Ma Y, Huang Y, Chen Y (2008) High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J Phys Chem C 112(45):17554–17558
Abazari R, Mahjoub AR, Ataei F, Morsali A, Carpenter-Warren CL, Mehdizadeh K, Slawin AM (2018) Chitosan immobilization on bio-MOF nanostructures: a biocompatible pH-responsive nanocarrier for doxorubicin release on MCF-7 cell lines of human breast cancer. Inorg Chem 57(21):13364–13379
Nie S, Hsiao WW, Pan W, Yang Z (2011) Thermoreversible Pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: in vitro drug release, cell cytotoxicity, and uptake studies. Int J Nanomed 6:151
Zheng H, Zhang Y, Liu L, Wan W, Guo P, Nyström AM, Zou X (2016) One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc 138(3):962–968
Lei B, Wang M, Jiang Z, Qi W, Su R, He Z (2018) Constructing redox-responsive metal–organic framework nanocarriers for anticancer drug delivery. ACS Appl Mater Interfaces 10(19):16698–16706
Chen X, Tong R, Shi Z, Yang B, Liu H, Ding S, Wang X, Lei Q, Wu J, Fang W (2018) MOF nanoparticles with encapsulated autophagy inhibitor in controlled drug delivery system for antitumor. ACS Appl Mater Interfaces 10(3):2328–2337
Shamsipur M, Molaabasi F, Sarparast M, Roshani E, Vaezi Z, Alipour M, Molaei K, Naderi-Manesh H, Hosseinkhani S (2018) Photoluminescence mechanisms of dual-emission fluorescent silver nanoclusters fabricated by human hemoglobin template: from oxidation-and aggregation-induced emission enhancement to targeted drug delivery and cell imaging. ACS Sustain Chem Eng 6(8):11123–11137
Yang Y, Hu Q, Zhang Q, Jiang K, Lin W, Yang Y, Cui Y, Qian G (2016) A large capacity cationic metal–organic framework nanocarrier for physiological pH responsive drug delivery. Mol Pharm 13(8):2782–2786
Zhang F-M, Dong H, Zhang X, Sun X-J, Liu M, Yang D-D, Liu X, Wei J-Z (2017) Postsynthetic modification of ZIF-90 for potential targeted codelivery of two anticancer drugs. ACS Appl Mater Interfaces 9(32):27332–27337
Xing K, Fan R, Wang F, Nie H, Du X, Gai S, Wang P, Yang Y (2018) Dual-stimulus-triggered programmable drug release and luminescent ratiometric pH sensing from chemically stable biocompatible zinc metal-organic framework. ACS Appl Mater Interfaces 10(26):22746–22756
Gao PF, Zheng LL, Liang LJ, Yang XX, Li YF, Huang CZ (2013) A new type of pH-responsive coordination polymer sphere as a vehicle for targeted anticancer drug delivery and sustained release. J Mater Chem B 1(25):3202–3208
Kundu T, Mitra S, Patra P, Goswami A, Díaz Díaz D, Banerjee R (2014) Mechanical downsizing of a gadolinium (III)-based metal–organic framework for anticancer drug delivery. Chemistry A Eur J 20(33):10514–10518
Acknowledgements
The authors gratefully acknowledge the support of this work by Iran University of Science and Technology and Motamed Cancer Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supporting information:
Detailed description on procedures, DOX loading in Cu-TCPP MOF and CuG nanocomposites, and further characterizations, including PXRD pattern of GO, Elemental mapping of CuG1, zeta potential results for the Cu-TCPP MOF, CuG1 and CuG1-DOX, several mathematical models (Higuchi, zero order, first order and Korsmeyer–Peppas) and different kinetics parameters of DOX release from CuG1, Kinetics of DOX release from CuG1, in vitro DOX release of the Cu-TCPP MOF-DOX and CuG1-DOX at various pH with additional microscopic images are presented in supporting information (PDF 2068 KB)
Rights and permissions
About this article
Cite this article
Gharehdaghi, Z., Rahimi, R., Naghib, S.M. et al. Cu (II)-porphyrin metal–organic framework/graphene oxide: synthesis, characterization, and application as a pH-responsive drug carrier for breast cancer treatment. J Biol Inorg Chem 26, 689–704 (2021). https://doi.org/10.1007/s00775-021-01887-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-021-01887-3