Abstract
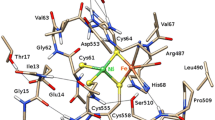
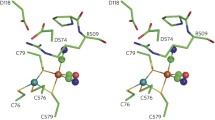
[FeFe] hydrogenases are H2-evolving enzymes that feature a diiron cluster in their active site (the [2Fe]H cluster). One of the iron atoms has a vacant coordination site that directly interacts with H2, thus favoring its splitting in cooperation with the secondary amine group of a neighboring, flexible azadithiolate ligand. The vacant site is also the primary target of the inhibitor O2. The [2Fe]H cluster can span various redox states. The active-ready form (Hox) attains the FeIIFeI state. States more oxidized than Hox were shown to be inactive and/or resistant to O2. In this work, we used density functional theory to evaluate whether azadithiolate-to-iron coordination is involved in oxidative inhibition and protection against O2, a hypothesis supported by recent results on biomimetic compounds. Our study shows that Fe–N(azadithiolate) bond formation is favored for an FeIIFeII active-site model which disregards explicit treatment of the surrounding protein matrix, in line with the case of the corresponding FeIIFeII synthetic system. However, the study of density functional theory models with explicit inclusion of the amino acid environment around the [2Fe]H cluster indicates that the protein matrix prevents the formation of such a bond. Our results suggest that mechanisms other than the binding of the azadithiolate nitrogen protect the active site from oxygen in the so-called H inactox state.







Similar content being viewed by others
Notes
Phosphorous-bound methyl and phenyl groups were disregarded in such an analysis, given the flatness of the energy landscape associated with their rotation.
In the electronic supplementary material, we also present results for alternative medium-sized models that include either the side chain of Cys178 (a residue that is capable of establishing a hydrogen bond with the amine group of the pendant as shown in Scheme 1; model denomination cys M, Fig. S3) or a fragment of the protein backbone surrounding Ala109 (a residue that is hydrogen-bonded to the Fep-bound CN– group; model termed ala M, Fig. S4). In both the cys M model and the ala M model, the Hoverox state featuring an Fed–N bond is stabler than the alternative conformation in which such a bond is absent. In the Hox state of both cys M and ala M, the Fed–N bond can form, but the resulting conformations are 2.7 and 4.1 kcal mol–1 less stable than the alternative isomers without such a bond (see the electronic supplementary material for further details).
References
Lubitz W, Reijerse E, van Gastel M (2007) Chem Rev 107:4331–4365. doi:10.1021/Cr050186q
Tard C, Pickett CJ (2009) Chem Rev 109:2245–2274. doi:10.1021/Cr800542q
Nicolet Y, Piras C, Legrand P, Hatchikian CE, Fontecilla-Camps JC (1999) Structure 7:13–23. doi:10.1016/S0969-2126(99)80005-7
Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC (1998) Science 282:1853–1858
Pandey AS, Harris TV, Giles LJ, Peters JW, Szilagyi RK (2008) J Am Chem Soc 130:4533–4540. doi:10.1021/ja711187e
Bruschi M, Greco C, Kaukonen M, Fantucci P, Ryde U, De Gioia L (2009) Angew Chem Int Ed 48:3503–3506. doi:10.1002/anie.200900494
Adamska ASA, Lambertz C, Rüdiger O, Happe T, Reijerse E, Lubitz W (2012) Angew Chem Int Ed 51:11458–11462
Fan HJ, Hall MB (2001) J Am Chem Soc 123:3828–3829. doi:10.1021/ja004120i
Greco C, Bruschi M, De Gioia L, Ryde U (2007) Inorg Chem 46:5911–5921. doi:10.1021/Ic062320a
Lemon BJ, Peters JW (1999) Biochemistry 38:12969–12973
Baffert C, Bertini L, Lautier T, Greco C, Sybirna K, Ezanno P, Etienne E, Soucaille P, Bertrand P, Bottin H, Meynial-Salles I, De Gioia L, Leger C (2011) J Am Chem Soc 133:2096–2099. doi:10.1021/Ja110627b
Bruska MK, Stiebritz MT, Reiher M (2011) J Am Chem Soc 133:20588–20603. doi:10.1021/Ja209165r
Stiebritz MT, Reiher M (2009) Inorg Chem 48:7127–7140. doi:10.1021/Ic9002127. Erratum in: Stiebritz MT, Reiher M (2010) Inorg Chem 49:8645. doi10.1021/ic1015737
Stripp ST, Goldet G, Brandmayr C, Sanganas O, Vincent KA, Haumann M, Armstrong FA, Happe T (2009) Proc Natl Acad Sci USA 106:17331–17336. doi:10.1073/pnas.0905343106
Baffert C, Demuez M, Cournac L, Burlat B, Guigliarelli B, Bertrand P, Girbal L, Leger C (2008) Angew Chem Int Ed 47:2052–2054. doi:10.1002/anie.200704313
Wait AF, Brandmayr C, Stripp ST, Cavazza C, Fontecilla-Camps JC, Happe T, Armstrong FA (2011) J Am Chem Soc 133:1282–1285. doi:10.1021/Ja110103p
Foster CE, Kramer T, Wait AF, Parkin A, Jennings DP, Happe T, McGrady JE, Armstrong FA (2012) J Am Chem Soc 134:7553–7557. doi:10.1021/Ja302096r
Ryde U, Greco C, De Gioia L (2010) J Am Chem Soc 132:4512. doi:10.1021/ja909194f
Nicolet Y, de Lacey AL, Vernede X, Fernandez VM, Hatchikian EC, Fontecilla-Camps JC (2001) J Am Chem Soc 123:1596–1601. doi:10.1021/ja0020963
Silakov A, Wenk B, Reijerse E, Lubitz W (2009) Phys Chem Chem Phys 11:6592–6599. doi:10.1039/B905841a
Erdem OF, Schwartz L, Stein M, Silakov A, Kaur-Ghumaan S, Huang P, Ott S, Reijerse EJ, Lubitz W (2011) Angew Chem Int Ed 50:1439–1443. doi:10.1002/anie.201006244
Greco C, Bruschi M, Heimdal J, Fantucci P, De Gioia L, Ryde U (2007) Inorg Chem 46:7256–7258. doi:10.1021/Ic701051h
Greco C, Bruschi M, Fantucci P, Ryde U, De Gioia L (2011) Chem Eur J 17:1954–1965. doi:10.1002/chem.201001493
Knorzer P, Silakov A, Foster CE, Armstrong FA, Lubitz W, Happe T (2012) J Biol Chem 287:1489–1499. doi:10.1074/jbc.M111.305797
Fontecilla-Camps JC, Volbeda A, Cavazza C, Nicolet Y (2007) Chem Rev 107:4273–4303. doi:10.1021/cr050195z
Roseboom W, De Lacey AL, Fernandez VM, Hatchikian EC, Albracht SPJ (2006) J Biol Inorg Chem 11:102–118. doi:10.1007/s00775-005-0040-2
Albracht SPJ, Roseboom W, Hatchikian EC (2006) J Biol Inorg Chem 11:88–101. doi:10.1007/s00775-005-0039-8
Vanderwesten HM, Mayhew SG, Veeger C (1978) FEBS Lett 86:122–126. doi:10.1016/0014-5793(78)80112-4
Pereira AS, Tavares P, Moura I, Moura JJG, Huynh BH (2001) J Am Chem Soc 123:2771–2782. doi:10.1021/Ja003176+
Huynh BH, Czechowski MH, Kruger HJ, Dervartanian DV, Peck HD, Legall J (1984) Proc Natl Acad Sci USA 81:3728–3732. doi:10.1073/pnas.81.12.3728
Liu ZP, Hu P (2002) J Am Chem Soc 124:5175–5182. doi:10.1021/Ja0118690
Cao ZX, Hall MB (2001) J Am Chem Soc 123:3734–3742. doi:10.1021/Ja000116v
Vincent KA, Parkin A, Lenz O, Albracht SPJ, Fontecilla-Camps JC, Cammack R, Friedrich B, Armstrong FA (2005) J Am Chem Soc 127:18179–18189. doi:10.1021/Ja055160v
Vandijk C, Vanberkelarts A, Veeger C (1983) FEBS Lett 156:340–344
Silakov A, Kamp C, Reijerse E, Happe T, Lubitz W (2009) Biochemistry 48:7780–7786. doi:10.1021/Bi9009105
Stiebritz MT, Reiher M (2012) Chem Sci 3:1739–1751. doi:10.1039/c2sc01112c
Olsen MT, Rauchfuss TB, Wilson SR (2010) J Am Chem Soc 132:17733–17740. doi:10.1021/Ja103998v
Vincent KA, Parkin A, Armstrong FA (2007) Chem Rev 107:4366–4413. doi:10.1021/Cr050191u
Moro G, Bonati L, Bruschi M, Cosentino U, De Gioia L, Fantucci P, Pandini A, Papaleo E, Pitea D, Saracino GAA, Zampella G (2007) Theor Chem Acc 117:723–741. doi:10.1007/s00214-006-0203-4
Cosentino U, Pitea D, Moro G, Saracino GAA, Villa A (2009) Phys Chem Chem Phys 11:3943–3950. doi:10.1039/B902049g
Cosentino U, Moro G, Pitea D, Villa A, Fantucci PC, Maiocchi A, Uggeri F (1998) J Phys Chem A 102:4606–4614. doi:10.1021/Jp973271d
Siegbahn PEM, Tye JW, Hall MB (2007) Chem Rev 107:4414–4435. doi:10.1021/Cr050185y
Vastine BA, Hall MB (2009) Coord Chem Rev 253:1202–1218. doi:10.1016/j.ccr.2008.07.015
Reiher M (2009) Chimia 63:140–145. doi:10.2533/chimia.2009.140
Ahlrichs R, Bar M, Haser M, Horn H, Kolmel C (1989) Chem Phys Lett 162:165–169. doi:10.1016/0009-2614(89)85118-8
Becke AD (1988) Phys Rev A 38:3098–3100. doi:10.1103/PhysRevA.38.3098
Perdew JP (1986) Phys Rev B 33:8822–8824. doi:10.1103/PhysRevB.33.8822
Schafer A, Huber C, Ahlrichs R (1994) J Chem Phys 100:5829–5835
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297–3305. doi:10.1039/B508541a
Eichkorn K, Treutler O, Ohm H, Haser M, Ahlrichs R (1995) Chem Phys Lett 240:283–289. doi:10.1016/0009-2614(95)00621-A
Eichkorn K, Weigend F, Treutler O, Ahlrichs R (1997) Theor Chem Acc 97:119–124. doi:10.1007/s002140050244
Klamt A (1995) J Phys Chem 99:2224–2235. doi:10.1021/J100007a062
Noodleman L, Norman JG (1979) J Chem Phys 70:4903–4906. doi:10.1063/1.437369
Noodleman L (1981) J Chem Phys 74:5737–5743. doi:10.1063/1.440939
Greco C, Fantucci P, Ryde U, de Gioia L (2011) Int J Quantum Chem 111:3949–3960. doi:10.1002/Qua.22849
Yu L, Greco C, Bruschi M, Ryde U, De Gioia L, Reiher M (2011) Inorg Chem 50:3888–3900. doi:10.1021/Ic102039z
Becke AD (1993) J Chem Phys 98:1372–1377. doi:10.1063/1.464304
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Greco C, De Gioia L (2011) Inorg Chem 50:6987–6995. doi:10.1021/Ic200297d
Matito E, Sola M (2009) Coord Chem Rev 253:647–665. doi:10.1016/j.ccr.2008.10.003
Francuski BM, Novakovic SB, Bogdanovic GA (2011) CrystEngComm 13:3580–3591. doi:10.1039/C0ce00760a
Greco C, Silakov A, Bruschi M, Ryde U, De Gioia L, Lubitz W (2011) Eur J Inorg Chem 2011:1043–1049. doi:10.1002/ejic.201001058
Acknowledgments
Financial support of this work from the Cluster of Excellence “Unifying Concepts in Catalysis” (Berlin) is gratefully acknowledged; We acknowledge the CINECA award under the ISCRA initiative, for the availability of high performance computing resources and support. C.L., F.V., and C.B. acknowledge the CNRS, Aix-Marseille Université, and the ANR (ANR-12-BS08-0014) for funding.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyake, T., Bruschi, M., Cosentino, U. et al. Does the environment around the H-cluster allow coordination of the pendant amine to the catalytic iron center in [FeFe] hydrogenases? Answers from theory. J Biol Inorg Chem 18, 693–700 (2013). https://doi.org/10.1007/s00775-013-1014-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-013-1014-4