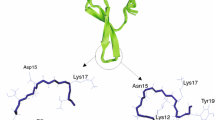
Abstract
Metal ions have been shown to play a critical role in β-amyloid (Aβ) neurotoxicity, thus prompting an intense investigation into the formation of metal–Aβ complexes. Isothermal titration calorimetry (ITC) has been widely used to determine binding constants (K) for a variety of metal–protein interactions, including those in metal–Aβ complexes. In this study, ITC was used to more fully quantify the thermodynamics (K, ΔG, ΔH, and TΔS) of Cu2+ binding to Aβ16, N-acetyl-Aβ16, Aβ28, N-acetyl-Aβ28, and Aβ28 variants (H6A, H13A, H14A) at pH 7.4 and 37 °C. After deconvolution of competing reactions, K for Aβ16 was found to be 1.1 (±0.13) × 109 and is in strong agreement with literature values measured under similar conditions. Further, a similar K value was obtained at two additional concentrations of competing ligand, suggesting that ternary complex formation is not significant. The acetylated peptide analogs reveal a marked decrease in the overall free energy upon binding, which is the result of less favorable enthalpic and entropic contributions. Circular dichroism spectroscopy shows conformational changes that are consistent with these results. Most importantly, data for Aβ28 variants lacking a potential Cu2+-binding histidine residue reveal that the overall free energy of binding remains constant, which is the result of entropy/enthalpy compensation. These data provide fundamental thermodynamic evidence for coordination plasticity in Cu2+ binding to Aβ and other intrinsically disordered peptides.







Similar content being viewed by others
Abbreviations
- Aβ:
-
β-Amyloid
- ACES:
-
N-(2-Acetomido)-2-aminoethanesulfonic acid
- AD:
-
Alzheimer’s disease
- CD:
-
Circular dichroism
- ITC:
-
Isothermal titration calorimetry
References
Anonymous (2011) Alzheimers Dement 7:1–63
Hebert L, Scherr P, Bienias J, Bennett D, Evans D (2003) Arch Neurol 60:1119–1122
Barnham KJ, Masters CL, Bush AI (2004) Nat Rev Drug Discov 3:205–214
Glenner GG (1981) Ann Pathol 1:105–108
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Proc Natl Acad Sci USA 82:4245–4249
Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Mullerhill B (1987) Nature 325:733–736
Beauchemin D, Kisilevsky R (1998) Anal Chem 70:1026
Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR (1998) J Neurol Sci 158:47–52
Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR (2003) Biochemistry 42:2768–2773
Tougu V, Tiiman A, Palumaa P (2011) Metallomics 3:250–261
Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI (2001) Neuron 30:665–676
Bush AI, Tanzi RE (2008) Neurotherapeutics 5:421–432
Faller P, Hureau C (2009) Dalton Trans 13:1080–1094
Miura T, Suzuki K, Kohata N, Takeuchi H (2000) Biochemistry 39:7024–7031
Curtain CC, Ali F, Volitakis I, Cherny RA, Norton RS, Beyreuther K, Barrow CJ, Masters CL, Bush AI, Barnham KJ (2001) J Biol Chem 276:20466–20473
Kowalik-Jankowska T, Ruta-Dolejsz M, Wisniewska K, Lankiewicz L (2001) J Inorg Biochem 86:535–545
Kowalik-Jankowska T, Ruta M, Wisniewska K, Lankiewicz L (2003) J Inorg Biochem 95:270–282
Syme CD, Nadal RC, Rigby SEJ, Viles JH (2004) J Biol Chem 279:18169–18177
Karr JW, Kaupp LJ, Szalai VA (2004) J Am Chem Soc 126:13534–13538
Karr JW, Akintoye H, Kaupp LJ, Szalai VA (2005) Biochemistry 44:5478–5487
Karr JW, Szalai VA (2007) J Am Chem Soc 129:3796–3797
Karr JW, Szalai VA (2008) Biochemistry 47:5006–5016
Guilloreau L, Damian L, Coppel Y, Mazarguil H, Winterhalter M, Faller P (2006) J Biol Inorg Chem 11:1024–1038
Minicozzi V, Stellato F, Comai M, Serra MD, Potrich C, Meyer-Klaucke W, Morante S (2008) J Biol Chem 283:10784–10792
Dorlet P, Gambarelli S, Faller P, Hureau C (2009) Angew Chem Int Ed 48:9273–9276
Drew SC, Noble CJ, Masters CL, Hanson GR, Barnham KJ (2009) J Am Chem Soc 131:1195–1207
Drew SC, Masters CL, Barnham KJ (2009) J Am Chem Soc 131:8760–8761
Garzon-Rodriguez W, Yatsimirsky AK, Glabe CG (1999) Bioorg Med Chem Lett 9:2243–2248
Tougu V, Karafin A, Palumaa P (2008) J Neurochem 104:1249–1259
Sarell CJ, Syme CD, Rigby SEJ, Viles JH (2009) Biochemistry 48:4388–4402
Hong L, Bush WD, Hatcher LQ, Simon J (2008) J Phys Chem B 112:604–611
Hatcher LQ, Hong L, Bush WD, Carducci T, Simon JD (2008) J Phys Chem B 112:8160–8164
Hong L, Carducci TM, Bush WD, Dudzik CG, Millhauser GL, Simon JD (2010) J Phys Chem B 114:11261–11271
Rozga M, Kloniecki M, Dadlez M, Bal W (2010) Chem Res Toxicol 23:336–340
Rozga M, Protas AM, Jablonowska A, Dadlez M, Bal W (2009) Chem Commun 11:1374–1376
Rozga M, Bal W (2010) Chem Res Toxicol 23:298–308
Xiao Z, Wedd AG (2010) Nat Prod Rep 27:768–789
Wilcox DE (2008) Inorg Chim Acta 361:857–867
Grossoehme NE, Spuches AM, Wilcox DE (2010) J Biol Inorg Chem 15:1183–1191
Shen CL, Scott GL, Merchant F, Murphy RM (1993) Biophys J 65:2383–2395
Talmard C, Guilloreau L, Coppel Y, Mazarguil H, Faller P (2007) Chembiochem 8:163–165
Hong L, Simon JD (2011) Metallomics 3:262–266
Liu RT, McAllister C, Lyubchenko Y, Sierks MR (2004) J Neurosci Res 75:162–171
Gill SC, von Hippel PH (1989) Anal Biochem 182:319–326
Martell AE, Smith RM (2004) NIST Standard Reference Database 46. NIST critically selected stability constants of metal complexes, version 8.0. National Institute of Standards and Technology, Gaithersburg
Trapaidze A, Hureau C, Bal W, Winterhalter M, Faller P (2011) J Biol Inorg Chem 17:37–47
Atwood CS, Scarpa RC, Huang XD, Moir RD, Jones WD, Fairlie DP, Tanzi RE, Bush AI (2000) J Neurochem 75:1219–1233
Chen W, Liao Y, Yu H, Cheng IH, Chen Y (2011) J Biol Chem 286:9646–9656
Fawcett TG, Bernarducci EE, Kroghjespersen K, Schugar HJ (1980) J Am Chem Soc 102:2598–2604
Tsangaris JM, Martin RB (1970) J Am Chem Soc 92:4255–4260
Alies B, Eury H, Bijani C, Rechignat L, Faller P, Hureau C (2011) Inorg Chem 50:11192–11201
LeVine H (2002) Arch Biochem Biophys 404:106–115
Yu H, Ren J, Qu X (2008) Chembiochem 9:879–882
Shivers BD, Hilbich C, Multhaup G, Salbaum M, Beyreuther K, Seeburg PH (1988) EMBO J 7:1365–1370
Busciglio J, Yeh J, Yankner BA (1993) J Neurochem 61:1565–1568
Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NME, Romano DM, Hartshorn MA, Tanzi RE, Bush AI (1998) J Biol Chem 273:12817–12826
Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP (1991) Mol Brain Res 10:299–305
Gaggelli E, Grzonka Z, Kozlowski H, Migliorini C, Molteni E, Valensin D, Valensin G (2008) Chem Commun 3:341–343
Eury H, Bijani C, Faller P, Hureau C (2011) Angew Chem Int Ed 50:901–905
Acknowledgments
We would like to thank William E. Allen, Andrew Sargent, Andrew Morehead, and Colin Burns for their useful comments and advice. We thank Eli G. Hvastkovs for creating Scheme 2. We would also like to thank Nicholas E. Grossoehme and Dean E. Wilcox for advice on figures, extraction of buffer-independent binding constants, and other useful comments. We thank East Carolina University for the start-up funds that made this work possible. This article is dedicated to the memory of Francesa Spuches.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sacco, C., Skowronsky, R.A., Gade, S. et al. Calorimetric investigation of copper(II) binding to Aβ peptides: thermodynamics of coordination plasticity. J Biol Inorg Chem 17, 531–541 (2012). https://doi.org/10.1007/s00775-012-0874-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-012-0874-3