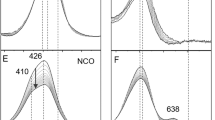
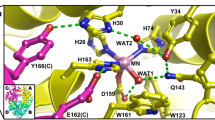
Abstract
Nickel-dependent superoxide dismutase (NiSOD) is a member of a class of metalloenzymes that protect aerobic organisms from the damaging superoxide radical (O2 ·−). A distinctive and fascinating feature of NiSOD is the presence of active-site nickel–thiolate interactions involving the Cys2 and Cys6 residues. Mutation of one or both Cys residues to Ser prevents catalysis of O2 ·−, demonstrating that both residues are necessary to support proper enzymatic activity (Ryan et al., J Biol Inorg Chem, 2010). In this study, we have employed a combined spectroscopic and computational approach to characterize three Cys-to-Ser (Cys → Ser) mutants (C2S, C6S, and C2S/C6S NiSOD). Similar electronic absorption and magnetic circular dichroism spectra are observed for these mutants, indicating that they possess nearly identical active-site geometric and electronic structures. These spectroscopic data also reveal that the Ni2+ ion in each mutant adopts a high-spin (S = 1) configuration, characteristic of a five- or six-coordinate ligand environment, as opposed to the low-spin (S = 0) configuration observed for the four-coordinate Ni2+ center in the native enzyme. An analysis of the electronic absorption and magnetic circular dichroism data within the framework of density functional theory computations performed on a series of five- and six-coordinate C2S/C6S NiSOD models reveals that the active site of each Cys → Ser mutant possesses an essentially six-coordinate Ni2+ center with a rather weak axial bonding interaction. Factors contributing to the lack of catalytic activity displayed by the Cys → Ser NiSOD mutants are explored.












Similar content being viewed by others
Abbreviations
- B3LYP:
-
Becke’s three-parameter hybrid functional for exchange combined with the Lee–Yang–Par correlation functional
- CD:
-
Circular dichroism
- Cys → Ser:
-
Cysteine to serine
- DFT:
-
Density functional theory
- EPR:
-
Electron paramagnetic resonance
- EXAFS:
-
Extended X-ray absorption fine structure
- HOMO:
-
Highest occupied molecular orbital
- INDO/S-CI:
-
Intermediate neglect of differential overlap/spectroscopic parameterization with configuration interaction
- LF:
-
Ligand field
- MCD:
-
Magnetic circular dichroism
- MO:
-
Molecular orbital
- NiSOD:
-
Nickel-dependent superoxide dismutase
- NiSODox :
-
Oxidized nickel-dependent superoxide dismutase
- NiSODred :
-
Reduced nickel-dependent superoxide dismutase
- SOD:
-
Superoxide dismutase
- TD-DFT:
-
Time-dependent density functional theory
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Imlay JA (2003) Annu Rev Microbiol 57:395–418
Midorikawa K, Kawanishi S (2001) FEBS Lett 495:187–190
Valentine JS, Wertz DL, Lyons TJ, Liou L-L, Goto JJ, Gralla EB (1998) Curr Opin Chem Biol 2:253–262
Moller IM (2001) Annu Rev Plant Physiol Plant Mol Biol 52:561–591
Simonian NA, Coyle JT (1996) Rev Pharmacol Toxicol 36:83–106
Beyer W, Imlay J, Fridovich I (1991) Prog Nucl Acid Res Mol Biol 40:221–253
Brown RHJ (1997) Cold Spring Harb Monogr Ser 34:569–585
Chatterjee S, Fisher AB (2005) In: Bagchi D, Preuss HG (eds) Phytopharmaceuticals in cancer chemoprevention. CRC Press, Boca Raton, pp 171–186
Halliwell B (1990) Methods Enzymol 186:1–85
Halliwell B (1995) In: Valentine JS, Foote CS, Greenberg A, Liebman JF (eds) Active oxygen in biochemistry. Blackie, New York, pp 313–335
Jenner P, Olanow CW (1998) Ann Neurol 44:S72–S84
Passi S, Ricci R, Aleo E, Cocchi M (2005) Prog Nutr 7:3–22
Trotti D, Rolfs A, Danbolt NC, Brown JRH, Hediger MA (1999) Nat Neurosci 2:427–433
Wallace DC (1992) Science 256:628–632
Valentine JS, Hart PJ (2003) Proc Natl Acad Sci USA 100:3617–3622
Kurtz DM (2004) Acc Chem Res 37:902–908
Miller AF (2004) Curr Opin Chem Biol 8:162–168
Miller AF, Sorkin DL (1997) Commun Mol Cell Biophys 9:1–48
Fridovich I (1995) Annu Rev Biochem 64:97–112
Fridovich I, William JL, Lane MD (2004) Encyclopedia of biological chemistry. Elsevier, New York, pp 135–138
Youn HD, Kim EJ, Roe JH, Hah YC, Kang SO (1996) Biochem J 318:889–896
Youn HD, Youn H, Lee JW, Yim YI, Lee JK, Hah YC, Kang SO (1996) Arch Biochem Biophys 334:341–348
Palenik B, Brahamsha B, Larimer FW, Land M, Hauser L, Chain P, Lamerdin J, Regala W, Allen EE, McCarren J, Paulsen I, Dufresne A, Partensky F, Webb EA, Waterbury J (2003) Nature 424:1037–1042
Barondeau DP, Kassmann CJ, Bruns CK, Tainer JA, Getzoff ED (2004) Biochemistry 43:8038–8047
Wuerges J, Lee JW, Yim YI, Yim HS, Kang SO, Carugo KD (2004) Proc Natl Acad Sci USA 101:8569–8574
Hart PJ, Balbirnie MM, Ogihara NL, Nersissian AM, Weiss MS, Valentine JS, Eisenberg D (1999) Biochemistry 38:2167–2178
Doukov TI, Iverson TM, Seravalli J, Ragsdale SW, Drennan CL (2002) Science 298:567–572
Kovacs JA (2004) Chem Rev 104:825–848
Chan Chung KC, Cao L, Dias AV, Pickering IJ, George GN, Zamble DB (2008) J Am Chem Soc 130:14056–14057
Harford C, Sarkar B (1997) Acc Chem Res 30:123–130
Lanzilotta WN, Schuller DJ, Thorsteinsson MV, Kerby RL, Roberts GP, Poulos TL (2000) Nat Struct Biol 7:876–880
Chohan BS, Maroney MJ (2006) Inorg Chem 45:1906–1908
Farmer PJ, Reibenspies JH, Lindahl PA, Darensbourg MY (1993) J Am Chem Soc 115:4665–4674
Farmer PJ, Solouki T, Mills DK, Soma T, Russell DH, Reibenspies JH, Darensbourg MY (1992) J Am Chem Soc 114:4601–4605
Grapperhaus CA, Darensbourg MY (1998) Acc Chem Res 31:451–459
Grapperhaus CA, Mullins CS, Kozlowski PM, Mashuta MS (2004) Inorg Chem 43:2859–2866
Green KN, Brothers SM, Jenkins RM, Carson CE, Grapperhaus CA, Darensbourg MY (2007) Inorg Chem 46:7536–7544
Kaasjager VE, Bouwman E, Gorter S, Reedijk J, Grapperhaus CA, Reibenspies JH, Smee JJ, Darensbourg MY, Derecskei-Kovacs A, Thomson LM (2002) Inorg Chem 41:1837–1844
Kumar M, Colpas GJ, Day RO, Maroney MJ (1989) J Am Chem Soc 111:8323–8325
Bellefeuille JA, Grapperhaus CA, Derecskei-Kovacs A, Reibenspies JH, Darensbourg MY (2000) Inorg Chim Acta 300–302:73–81
Smee JJ, Miller ML, Grapperhaus CA, Reibenspies JH, Darensbourg MY (2001) Inorg Chem 40:3601–3605
Allan CB, Davidson G, Choudhury SB, Gu Z, Bose K, Day RO, Maroney MJ (1998) Inorg Chem 37:4166–4167
Fiedler AT, Bryngelson PA, Maroney MJ, Brunold TC (2005) J Am Chem Soc 127:5449–5462
Neupane KP, Shearer J (2006) Inorg Chem 45:10552–10566
Shearer J, Dehestani A, Abanda F (2008) Inorg Chem 47:2649–2660
Maroney MJ (1999) Curr Opin Chem Biol 3:188–199
Volbeda A (1995) Nature 373:580–587
Dobbek H, Svetlitchnyi V, Gremer L, Huber R, Meyer O (2001) Science 293:1281–1285
Ermler U, Grabarse W, Shima S, Goubeaud M, Thauer RK (1997) Science 278:1457–1462
Heddle J, Scott DJ, Unzai S, Park S-Y, Tame JRH (2003) J Biol Chem 278:50322–50329
Jabri E, Carr MB, Hausinger RP, Karplus PA (1995) Science 268:998–1004
Song HK, Mulrooney SB, Huber R, Hausinger RP (2001) J Biol Chem 276:49359–49364
Watt RK, Ludden PW (1999) Cell Mol Life Sci 56:604–625
Choudhury SB, Lee JW, Davidson G, Yim YI, Bose K, Sharma ML, Kang SO, Cabelli DE, Maroney MJ (1999) Biochemistry 38:3744–3752
Herbst RW, Guce A, Bryngelson PA, Higgins KA, Rynan KC, Cabelli DE, Garman SC, Maroney MJ (2009) Biochemistry 48:3354–3369
Barrette WC, Sawyer DT, Fee JA, Asada K (1983) Biochemistry 22:624–627
Krueger HJ, Holm RH (1987) Inorg Chem 26:3645–3647
Krueger HJ, Peng G, Holm RH (1991) Inorg Chem 30:734–742
Ray D, Pal S, Chakravorty A (1986) Inorg Chem 25:2674–2677
Jackson TA, Brunold TC (2004) Acc Chem Res 37:461–470
Xie J, Yikilmaz E, Miller AF, Brunold TC (2002) J Am Chem Soc 124:3769–3774
Tietze D, Breitzke H, Imhof D, Kothe E, Weston J, Buntkowsky G (2009) Chem Eur J 15:517–523
Ryan KC, Johnson OE, Brunold TC, Johnson MK (2010) J Biol Inorg Chem. doi:10.1007/s00775-010-0645-y
Park IS, Michel LO, Pearson MA, Jabri E, Karplus PA, Wang SK, Dong J, Scott RA, Koehler BP, Johnson MK, Hausinger RP (1996) J Biol Chem 271:18632–18637
Baerends EJ, Ellis DE, Ros P (1973) Chem Phys 2:41–51
Guerra CF, Snijders JG, te Velde G, Baerends EJ (1998) Theor Chem Acc 99:391–403
te Velde G, Baerends EJ (1992) J Comput Phys 99:84–98
te Velde G, Bickelhaupt FF, Baerends EJ, Guerra CF, Van Gisbergen SJA, Snijders JG, Ziegler T (2001) J Comput Chem 22:931–967
Versluis L, Ziegler T (1988) J Chem Phys 88:322–328
Vosko SH, WIlk L, Nusair M (1980) Can J Phys 58:1200
Becke AD (1993) J Chem Phys 98:5648–5652
Perdew JP (1986) Phys Rev B 33:8822–8824
Neese F (2005) ORCA 2.4. Max-Planck-Institut für Bioanorganische Chemie. Mülheim an der Ruhr
Ridley J, Zerner MC (1973) Theor Chim Acta 32:111
Zerner MC, Loew GH, Kirchner RF, Muellerwesterhoff UT (1980) J Am Chem Soc 102:589–599
Bacon AD, Zerner MC (1979) Theor Chim Acta 53:21–54
Becke AD (1993) J Chem Phys 98:1372–1377
Lee C, Yang W, Parr RG (1988) Phys Rev B 37
Schafer A, Horn H, Achlrichs R (1992) J Chem Phys 97:2571–2577
Weigand F, Haeser M (1997) Theor Chem Acc 97:331
Schafer A, Huber C, Achlrichs R (1994) J Chem Phys 100:5829–5835
Laaksonen L (1992) J Mol Graph 10:33–34
Boge EM, Freyberg DP, Kokot E, Mockler GM, Sinn E (1977) Inorg Chem 16:1655–1660
Hammes BS, Carrano CJ (1999) Inorg Chem 38:3562–3568
Martin LY, Sperati CR, Busch DH (1977) J Am Chem Soc 99:2968–2981
Cameron JH, McCullough KJ (1990) J Chem Soc Dalton Trans 935–941
Lever ABP (1984) Inorganic Electronic Spectroscopy. Elsevier, New York, pp 507–530
Johnson MK (2000) In: Que L Jr (ed) Physical methods in bioinorganic chemistry: spectroscopy and magnetism. University Science Books, Sausalito, pp 253–259
Desrochers PJ, Telser J, Zvyagin SA, Ozarowski A, Krzystek J, Vicic DA (2006) Inorg Chem 45:8930–8941
Steenkamp PJ (1987) Inorg Chim Acta 132:27–31
Kolis JW, Hamilton DE, Kildahl NK (1979) Inorg Chem 18:1826–1831
Sacconi L, Ciampolini M, Speroni GP (1965) J Am Chem Soc 87:3102–3106
Sacconi L, Nannelli P, Nardi N, Campigli U (1965) Inorg Chem 4:943–949
Sacconi L, Orioli PL, Di Vaira M (1996) Inorg Chem 4:943–949
Arakawa S, Nozawa T, Hatano M (1974) Bull Chem Soc Jpn 47:2643–2646
Harding MJ, Mason SF, Robbins DJ, Thomson AJ (1971) J Chem Soc A 19:3058–3062
Harding MJ, Mason SF, Robbins DJ, Thomson AJ (1971) J Chem Soc A 19:3047–3058
Hormann E, Riesen PC, Neuburger M, Zehnder M, Kaden TA (1996) Helv Chim Acta 79:235–243
Katô H, Yorita K, Kato Y (1979) Bull Chem Soc Jpn 52:2465–2473
Kobayashi H, Bohdan K-D (1972) Bull Chem Soc Jpn 45:2485–2490
Kobayashi N, Xizhang Z, Tetsuo O (1987) J Chem Soc Dalton Trans 1801–1803
Scheiner AF, Hamm DJ (1973) Inorg Chem 12:2037–2048
Kiick KL (1991) Chemistry. University of Georgia, Athens, p 180
Averill DF, Legg JI, Smith DL (1971) Inorg Chem 11:2344–2349
Fukuda Y, Yamagata K, Kaden TA (1987) Bull Chem Soc Jpn 60:3569–3574
Ohtsu H, Tanaka K (2003) Inorg Chem 43:3024–3030
Ohtsu H, Tanaka K (2005) Chem Eur J 11:3420–3426
Neupane KP, Gearty K, Francis A, Shearer J (2007) J Am Chem Soc 129:14605–14618
Battaglia LP, Berzolla PG, Corradi AB, Pelizzi C, Pelosi G, Solinas C (1992) J Chem Soc Dalton Trans 3089–3095
Dobrzynska D, Jerzykiewicz LB, Duczmal M, Wojciechowska A, Jablonska K, Palus J, Ozarowski A (2006) Inorg Chem 45:10479–10486
Du Q-Y, Li Y-P, Xin L-Y, Han M-L (2005) Z Kristallogr NCS 220:539–540
Ihara Y, Satake Y, Fujimoto Y, Senda H, Suzuki M, Uehara A (1991) Bull Chem Soc Jpn 64:2349–2352
Ide DMM, Norman RE (2007) Acta Crystallogr E 63:m558–m560
Atkinson IM, Byriel KA, Chia PSK, Kennard CHL, Leong AJ, Lindoy LF, Lowe MP, Mahendran S, Smith G, Wei G (1998) Aust J Chem 51:985–991
Thulstrup PN, Broge L, Larsen E, Springborg J (2003) J Chem Soc Dalton Trans 3199–3204
Xiao Z, Lavery MJ, Ayhan M, Scrofani SDB, Wilce MCJ, Guss JM, Tregloan PA, George GN, Wedd AG (1998) J Am Chem Soc 120:4135–4150
Pons J, Chadghan A, Casabo J, Alvarez-Larena A, Piniella JF, Solans X, Font-Bardia M, Ros J (2001) Polyhedron 20:1029–1035
Elerman Y, Kabak M (1993) Acta Crystallogr C 49:1905–1906
Nielson DO, Larsen ML, Willet RD, Legg JI (1971) J Am Chem Soc 93:5079–5082
Shintani N, Sone K, Fukuda Y, Ohashi Y (1991) Bull Chem Soc Jpn 64:252–258
Calatayud ML, Castro I, Sletten J, Cano J, Lloret F, Faus J, Julve M, Seitz G, Mann K (1996) Inorg Chem 35:2858–2865
Stranger R, McMahon KL, Gahan LR, Bruce JI, Hambley TW (1997) Inorg Chem 36:3466–3475
Cha M, Gatlin CL, Critchlow SC, Kovacs JA (2002) Inorg Chem 32:5868–5877
Chohan BS, Shoner SC, Kovacs JA, Maroney MJ (2004) Inorg Chem 43:7726–7734
Gomes L, Pereira E, de Castro B (2000) J Chem Soc Dalton Trans 1373–1379
Dutton JC, Fallon GD, Murray KS (1990) Chem Lett 983–986
Lipscomb WN, Strater N (1996) Chem Rev 96:2375–2434
Ruth-Gomis F-X, Grams F, Yiallouros I, Nar H, Kusthardt U, Zwilling R, Bode W, Stocker W (1994) J Biol Chem 269:17111–17117
Lee MH, Pankratz HS, Wang S, Scott RA, Finnegan MG, Johnson MK, Ippolito JA, Christianson DW, Hausinger RP (1993) Protein Sci 2:1042–1052
Carrington PE, Chivers PT, Al-Mjeni F, Sauer RT, Maroney MJ (2003) Nat Struct Biol 10:126–130
Davidson G, Clugston SL, Honek JF, Maroney MJ (2000) Inorg Chem 39:2962–2963
Haumann M, Porthun A, Buhrke T, Liebisch P, Meyer-Klaucke W, Friedrich B, Dau H (2003) Biochemistry 42:11004–11015
Mullins CS, Grapperhaus CA, Kozlowski PM (2006) J Biol Chem 11:617–625
Fox S, Wang Y, Silver A, Millar M (1990) J Am Chem Soc 112:3218–3220
Dey A, Jeffery SP, Darensbourg M, Hodgson KO, Hedman B, Solomon EI (2007) Inorg Chem 46:4989–4996
Acknowledgments
This work was supported by the National Institutes of Health (Grant GM 64631 to T.C.B.), the University of Wisconsin Chemical Biology Interface Training Grant from the National Institutes of Health (Grant T32 GM008505 to O.E.J.), and the National Science Foundation (Grant CHE-0809188 to M.J.M).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Johnson, O.E., Ryan, K.C., Maroney, M.J. et al. Spectroscopic and computational investigation of three Cys-to-Ser mutants of nickel superoxide dismutase: insight into the roles played by the Cys2 and Cys6 active-site residues. J Biol Inorg Chem 15, 777–793 (2010). https://doi.org/10.1007/s00775-010-0641-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-010-0641-2