Abstract
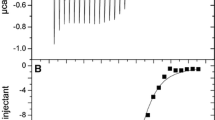
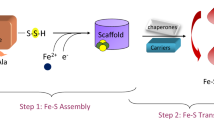
Recent work on the bacterial iron–sulfur cluster (isc) family of gene products, and eukaryotic homologs, has advanced the molecular understanding of cellular mechanisms of iron–sulfur cluster biosynthesis. Members of the IscS family are pyridoxyl-5′-phosophate dependent proteins that deliver inorganic sulfide during assembly of the [2Fe–2S] cluster on the IscU scaffold protein. Herein it is demonstrated through calorimetry, fluorescence, and protein stability measurements that Thermotoga maritima IscS forms a 1:1 complex with IscU in a concentration-dependent manner (K D varying from 6 to 34 μM, over an IscS concentration range of approximately 2–50 μM). Docking simulations of representative IscU and IscS proteins reveal critical contact surfaces at the N-terminal helix of IscU and a C-terminal loop comprising a chaperone binding domain. Consistent with the isothermal titration calorimetry results described here, an overall dominant contribution of charged surfaces with a change in the molar heat capacity of binding, ΔC p ~ 199.8 kcal K−1 mol−1, is observed that accounts for approximately 10% of the total accessible surface area at the binding interface. Both apo and holo IscUs and homologs were found to bind to IscS in an enthalpically driven reaction with comparable K D values. Both helix and loop regions are highly conserved among phylogenetically diverse organisms from a pool of archael, bacterial, fungal, and mammalian representatives.






Similar content being viewed by others
Abbreviations
- 1,5-IAEDANS:
-
5-((((2-Iodoacetyl)amino)ethyl)amino) naphthalene-1-sulfonic acid
- 5-BMF:
-
5-(Bromomethyl)fluorescein
- DTT:
-
Dithiothreitol
- FRET:
-
Fluorescence resonance energy transfer
- IPTG:
-
Isopropyl β-d-thiogalactopyranoside
- ITC:
-
Isothermal titration calorimetry
- PDB:
-
Protein Data Bank
- PLP:
-
Pyridoxyl 5′-phosphate
- PMSF:
-
Phenylmethylsulfonyl fluoride
- TCEP:
-
Tris(2-carboxyethyl)phosphine hydrochloride
References
Beinert H, Holm RH, Munck E (1997) Science 277:653–659
Beinert H (2000) J Biol Inorg Chem 5:2–15
Gutteridge JM, Rowley DA, Halliwell B (1982) Biochem J 206:605–609
Henle ES, Linn S (1997) J Biol Chem 272:19095–19098
Lauffer RB (1992) Iron and human disease. CRC Press, London
Gerber J, Lill R (2002) Mitochondrion 2:71–86
Takahashi Y, Tokumoto U (2002) J Biol Chem 277:28380–28383
Garland S, Hoff K, Vickery LE, Culotta VC (1999) J Mol Biol 294:897–907
Yoon T, Cowan JA (2003) J Am Chem Soc 125:6078–6084
Bencze KZ, Yoon T, Millán-Pacheco C, Bradley PB, Pastor N, Cowan JA, Stemmler TL (2007) Chem Commun 1798–1800
Yoon T, Cowan JA (2004) J Biol Chem 279:25943–25946
Gerber J, Muehlenhoff U, Lill R (2003) EMBO Rep 4:906–911
Ramazzotti A, Vanmansart V, Foury F (2004) FEBS Lett 557:215–220
Christen P, Mehta PK (2001) Chem Rev 1:436–447
Zheng L, White RH, Cash VL, Jack RF, Dean DR (1993) Proc Natl Acad Sci USA 90:2754–2758
Zheng L, White RH, Cash VL, Dean DR (1994) Biochemistry 33:4714–4720
Jaschkowitz K, Seidler A (2000) Biochemistry 39:3416–3423
Schneider D, Jaschkowitz K, Seidler A, Rogner M (2000) Indian J Biochem Biophys 37:441–446
Gubernator B, Seidler A, Rogner M, Szczepaniak A (2003) Protein Expr Purif 29:8–14
Kaiser JT, Clausen T, Bourenkow GP, Bartunik H-D, Steinbacher S, Huber R (2000) J Mol Biol 297:451–464
Cupp-Vickery JR, Urbina H, Vickery LE (2003) J Mol Biol 330:1049–1059
Mühlenhoff U, Balk J, Richhardt N, Kaiser JT, Sipos K, Kispal G, Lill R (2004) J Biol Chem 279:36906–36915
Li K, Tong W-H, Hughes RM, Rouault TA (2006) J Biol Chem 281:12344–12351
Bertini I, Cowan JA, Cristina DB, Luchinat C, Mansy SS (2003) J Mol Biol 331:907–924
Mansy SS, Wu G, Surerus KK, Cowan JA (2002) J Biol Chem 277:21397–21404
Foster MW, Mansy SS, Hwang J, Penner-Hahn JE, Surerus KK, Cowan JA (2000) J Am Chem Soc 122:6805–6806
Wu S-P, Wu G, Surerus K, Cowan JA (2002) Biochemistry 41:8876–8885
Siegel LM (1965) Anal Biochem 11:126–132
Wiseman T, Williston S, Brandts JF, Lin LN (1989) Anal Biochem 179:131–137
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Kluwer/Plenum, Dordrecht/New York
Jones S, Thorton JM (1996) Proc Natl Acad Sci USA 93:13–20
Chen R, Li L, Weng Z (2003) Protein 52:68–73
Comeau SR, Gatchell DW, Vajda S, Camacho CJ (2004) Bioinformatics 20:45–50
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M (1983) J Comput Chem 4:187–217
Urbina HD, Silberg JJ, Hoff KG, Vickery LE (2001) J Biol Chem 276:44521–44526
Yuvaniyama P, Agar JN, Cash VL, Johnson MK, Dean DR (2000) Proc Natl Acad Sci USA 97:599–604
Liu Y, Cowan JA (2007) Chem Commun 3192–3194
Mansy SS, Cowan JA (2004) Acc Chem Res 27:719–725
Muehlenhoff U, Gerber J, Richhardt N, Lill R (2003) EMBO J 22:4815–4825
Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK (2000) Biochemistry 39:7856–7862
Nuth M, Yoon T, Cowan JA (2002) J Am Chem Soc 124:8774–8775
Smith AD, Agar JN, Johnson KA, Frazzon J, Amster IJ, Dean DR, Johnson MK (2001) J Am Chem Soc 123:11103–11104
Cupp-Vickery JR, Peterson JC, Ta DT, Vickery LE (2004) J Mol Biol 342:1265–1278
Chervenak MC, Toone EJ (1994) J Am Chem Soc 116:10533–10539
Hoff K, Ta DT, Tapley TL, Silberg JJ, Vickery LE (2002) J Biol Chem 277:27353–27359
Acknowledgment
This work was supported by a grant the National Science Foundation, CHE-0111161 (J.A.C.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nuth, M., Cowan, J.A. Iron–sulfur cluster biosynthesis: characterization of IscU–IscS complex formation and a structural model for sulfide delivery to the [2Fe–2S] assembly site. J Biol Inorg Chem 14, 829–839 (2009). https://doi.org/10.1007/s00775-009-0495-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-009-0495-7