Abstract
Density functional theory calculations have been carried out to elucidate the mechanism of cyclohexane hydroxylation by three possible isomers of [FeIV(O)(N-R-N,N′,N′-tris(2-pyridylmethyl)ethane-1,2-diamine)]2+ (R is methyl or benzyl) (Klinker et al. in Angew Chem Int Ed 44:3690–3694, 2005). The calculations offer a mechanistic view and reveal the following features: (a) all the three isomers possess triplet ground states and low-lying quintet excited states, (b) the relative stability follows the order isomer A > isomer B > isomer C, in agreement with the conclusions of Klinker et al., (c) the theoretical investigations provide a rationale to explain the interconversion of the three isomers, (d) the reaction pathways of the C–H hydroxylation are initiated by a hydrogen-abstraction step, and (e) the three isomers react with cyclohexane via two-state-reactivity patterns on competing triplet and quintet spin-state surfaces. As such, in the gas phase, the relative reactivity exhibits the trend isomer B > isomer A, while at the highest level, B2//B1 with zero point energy and solvation corrections, the relative reactivity follows the order isomer B > isomer A > isomer C. Thus, the calculated reaction pathway shows that pyridine rings perpendicular to the Fe–O axis result in more reactive species, and a pyridine ring coordinated trans to the oxygen atom leads to the least reactive isomer.










Similar content being viewed by others
Notes
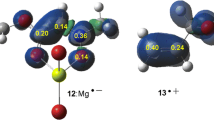
Isomer A contains two pyridine rings that eclipse the Fe–O axis and a third that is perpendicular to it. In isomer B one ring eclipses the Fe–O axis and two are perpendicular to it, whereas in isomer C two rings are perpendicular to the Fe–O axis and the other is coordinated trans to the oxygen atom [18].
[FeIV(O)(N4Py)]2+ persists for several days (t 1/2 ~ 60 h at 25 °C) and [FeIV(O)(Bn-TPEN)]2+ persists for several hours (t 1/2 ~ 6 h at 25 °C). The experimental data show that the free-energy barrier for [FeIV(O)(Bn-TPEN)]2+ with CH is 1 kcal/mol lower than the corresponding substrate with [FeIV(O)(N4Py)]2+ [13]. All in all, our calculations give the same conclusion as that of Kaizer et al. [13].
References
Que L Jr, Ho RYN (1996) Chem Rev 96:2607–2624
Solomon EI, Brunold TC, Davis M, Kemsley JN, Lee S-K, Lehnert N, Neese F, Skulan AJ, Yang Y-S, Zhou J (2000) Chem Rev 100:235–350
Costas M, Mehn MP, Jensen MP, Que L Jr (2004) Chem Rev 104:939–986
Price JC, Barr EW, Glass TE, Krebs C, Bollinger JM Jr (2003) J Am Chem Soc 125:13008–13009
Price JC, Barr EW, Tirupati B, Bollinger JM Jr, Krebs C (2003) Biochemistry 42:7497–7508
Proshlyakov DA, Henshaw TF, Monterosso GR, Ryle MJ, Hausinger RP (2004) J Am Chem Soc 126:1022–1023
Hoffart LM, Barr EW, Guyer RB, Bollinger JM Jr, Krebs C (2006) Proc Natl Acad Sci USA 103:14738–14743
Galonic DP, Barr EW, Walsh CT, Bollinger JM Jr, Krebs C (2007) Nat Chem Biol 3:113–116
Shan XP, Que L Jr (2006) J Inorg Biochem 100:421–433
Neese F (2006) J Inorg Biochem 100:716–726
Rohde J-U, In J-H, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L Jr (2003) Science 299:1037–1039
Lim MH, Rohde J-U, Stubna A, Bukoeski MR, Costas M, Ho RYN, Münck E, Nam W, Que L Jr (2003) Proc Natl Acad Sci USA 100:3665–3670
Kaizer J, Klinker EJ, Oh NY, Rohde J-U, Song WJ, Stubna A, Kim J, Münck E, Nam W, Que L Jr (2004) J Am Chem Soc 126:472–473
Kim SO, Sastri CV, Seo MS, Kim J, Nam W (2005) J Am Chem Soc 127:4178–4179
Oh NY, Suh Y, Park MJ, Seo MS, Kim J, Nam W (2005) Angew Chem Int Ed 44:4235–4239
Sastri CV, Park MJ, Ohta T, Jackson TA, Stubna A, Seo MS, Lee J, Kim J, Kitagawa T, Münck E, Que L Jr, Nam W (2005) J Am Chem Soc 127:12494–12495
Bukowski MR, Koehntop KD, Stubna A, Bominaar EL, Halfen JA, Münck E, Nam W, Que L Jr (2005) Science 310:1000–1002
Klinker EJ, Kaizer J, Brennessel WW, Woodrum NL, Cramer CJ, Que L Jr (2005) Angew Chem Int Ed 44:3690–3694
Sastri CV, Oh K, Lee YJ, Seo MS, Shin W, Nam W (2006) Angew Chem Int Ed 45:3992–3995
Krebs C, Fujimori DG, Walsh C, Bollinger JM Jr (2007) Acc Chem Res 40:484–492
Que L Jr (2007) Acc Chem Res 40:493–500
Decker A, Clay MD, Solomon EI (2006) J Inorg Biochem 100:697–706
Neidig ML, Decker A, Choroba OW, Huang FL, Kavana M, Moran GR, Spencer JB, Solomon EI (2006) Proc Natl Acad Sci USA 103:12966–12973
Decker A, Rohde J-U, Que L Jr, Solomon EI (2004) J Am Chem Soc 126:5378–5379
Pau MYM, Davis MI, Orville AM, Lipscomb JD, Solomon EI (2007) J Am Chem Soc 129:1944–1958
Schenk G, Pau MYM, Solomon EI (2004) J Am Chem Soc 126:505–515
Decker A, Solomon EI (2005) Angew Chem Int Ed 44:2252–2255
Decker A, Rohde J-U, Klinker EJ, Wong SD, Que L Jr, Solomon EI (2007) J Am Chem Soc 129:15983–15996
Decker A, Solomon EI (2005) Curr Opin Chem Biol 9:152–163
Shaik S, Hirao H, Kumar D (2007) Acc Chem Res 40:532–542
Johansson AJ, Blomberg MRA, Siegbahn PEM (2007) J Phys Chem C 111:12397–12406
Hirao H, Que L Jr, Nam W, Shaik S (2008) Chem Eur J 14:1740–1756
Hirao H, Kumar D, Que L Jr, Shaik S (2006) J Am Chem Soc 128:8590–8606
Kumar D, Hirao H, Que L Jr, Shaik S (2005) J Am Chem Soc 127:8026–8027
Hirao H, Chen H, Carvajal MA, Wang Y, Shaik S (2008) J Am Chem Soc 130:3319–3327
de Visser SP, Oh K, Han A-R, Nam W (2007) Inorg Chem 46:4632–4641
de Visser SP (2006) J Am Chem Soc 128:9813–9824
de Visser SP (2006) J Am Chem Soc 128:15809–15818
Balland V, Charlot A-F, Banse F, Girerd J-J, Mattioli TA, Bill E, Bartoli J-F, Battioni P, Mansuy D (2004) Eur J Inorg Chem 2:301–308
Martinho M, Banse F, Bartoli M-F, Mattioli TA, Battioni P, Horner O, Bourcier S, Girerd J-J (2005) Inorg Chem 44:9592–9596
Paine TK, Costas M, Kaizer J, Que L Jr (2006) J Biol Inorg Chem 11:272–276
Sastri CV, Seo MS, Park MJ, Kim KM, Nam W (2005) Chem Commun 1405–1407
You M, Seo MS, Kim KM, Nam W, Kim J (2006) Bull Korean Chem Soc 27:1140–1144
Sastri CV, Lee J, Oh K, Lee YJ, Lee J, Jackson TA, Ray K, Hirao H, Shin W, Halfen JA, Kim J, Que L Jr, Shaik S, Nam W (2007) Proc Natl Acad Sci USA 104:19181–19186
Rohde J-U, Que L Jr (2005) Angew Chem Int Ed 44:2255–2258
Nam W (2007) Acc Chem Res 40:522–531
Rohde J-U, Torelli S, Shan XP, Lim MH, Klinker EJ, Kaizer J, Chen K, Nam W, Que L Jr (2004) J Am Chem Soc 126:16750–16761
Park MJ, Lee J, Suh Y, Kim J, Nam W (2006) J Am Chem Soc 128:2630–2634
Nesheim JC, Lipscomb JD (1996) Biochemistry 35:10240–10247
Frisch MJ et al (2004) Gaussian 03, revision C.02. Gaussian, Wallingford
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1992) J Chem Phys 96:2155–2160
Becke AD (1992) J Chem Phys 97:9173–9177
Hay JP, Wadt WR (1985) J Chem Phys 82:299–310
Friesner RA, Murphy RB, Beachy MD, Ringlanda MN, Pollard WT, Dunietz BD, Cao YX (1999) J Phys Chem A 103:1913–1928
Melander L, Saunders WH Jr (1987) In: Reaction rates of isotopic molecules. Krieger, Malabar, chap 2
Kumar S, de Visser SP, Sharma PK, Cohen S, Shaik S (2004) J Am Chem Soc 126:1907–1920
de Visser SP (2006) J Biol Inorg Chem 11:168–178
Bassan A, Blomberg MRA, Siegbahn PEM (2003) Chem Eur J 9:4055–4067
Quinonero D, Morokuma K, Musaev DG, Mas-Balleste R, Que L Jr (2005) J Am Chem Soc 127:6548–6549
Handy NC, Cohen AJ (2001) Mol Phys 99:403–412
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Perdew JP, Burke K, Ernzerhof M (1997) Phys Rev Lett 78:1396–1396
Swart M, Ehlers AW, Lammertsma K (2004) Mol Phys 102:2467–2474
Groenhof AR, Swart M, Ehlers AW, Lammertsma K (2005) J Phys Chem A 109:3411–3417
Baker J, Pulay P (2002) J Chem Phys 117:1441–1449
Shaik S, Danovich D, Fiedler A, SchrÖder D, Schwarz H (1995) Helv Chem Acta 78:1393–1407
SchrÖder D, Shaik S, Schwarz H (2000) Acc Chem Res 33:139–145
Hirao H, Kumar D, Thiel W, Shaik S (2005) J Am Chem Soc 127:13007–13018
Mola J, Romero I, Rodríguez M, Bozoglian F, Poater A, Solà M, Parella T, Benet-Buchholz J, Fontrodona X, Llobet A (2007) Inorg Chem 46:10707–10716
Carter EA, Goddard WAIII (1988) J Phys Chem 92:5679–5683
Scott AP, Radom L (1996) J Phys Chem 100:16502–16513
Acknowledgments
This work was supported by NKBRSF(2007CB815202) and NSFC(20833008). Our deep appreciation goes to Sason Shaik and Hirao Hajime of the Jerusalem group for their generous help and to Edward I. Solomon and D. Wong Shaun of Stanford University for their useful advice.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, Y. & Han, K. Theoretical study of cyclohexane hydroxylation by three possible isomers of [FeIV(O)(R-TPEN)]2+: does the pentadentate ligand wrapping around the metal center differently lead to the different stability and reactivity?. J Biol Inorg Chem 14, 533–545 (2009). https://doi.org/10.1007/s00775-009-0468-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-009-0468-x