Abstract
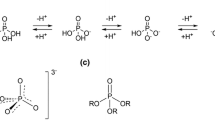
Phosphate esters play a central role in cellular energetics, biochemical activation, signal transduction and conformational switching. The structural homology of the borate anion with phosphate, combined with its ability to spontaneously esterify hydroxyl groups, suggested that phosphate ester recognition sites on proteins might exhibit significant affinity for nonenzymatically formed borate esters. 11B NMR studies and activity measurements on ribonuclease A (RNase A) in the presence of borate and several cytidine analogs demonstrate the formation of a stable ternary RNase A·3′-deoxycytidine–2′-borate ternary complex that mimics the complex formed between RNase A and a 2′-cytidine monophosphate (2′-CMP) inhibitor. Alternatively, no slowly exchanging borate resonance is observed for a ternary RNase A, borate, 2′-deoxycytidine mixture, demonstrating the critical importance of the 2′-hydroxyl group for complex formation. Titration of the ternary complex with 2′-CMP shows that it can displace the bound borate ester with a binding constant that is close to the reported inhibition constant of RNase A by 2′-CMP. RNase A binding of a cyclic cytidine-2′,3′-borate ester, which is a structural homolog of the cytidine-2′,3′-cyclic phosphate substrate, could also be demonstrated. The apparent dissociation constant for the cytidine-2′,3′-borate·RNase A complex is 0.8 mM, which compares with a Michaelis constant of 11 mM for cytidine-2′,3′-cyclic phosphate at pH 7, indicating considerably stronger binding. However, the value is 1,000-fold larger than the reported dissociation constant of the RNase A complex with uridine–vanadate. These results are consistent with recent reports suggesting that in situ formation of borate esters that mimic the corresponding phosphate esters supports enzyme catalysis.









Similar content being viewed by others
Abbreviations
- cCMB:
-
Cytidine-2′,3′-cyclic borate
- cCMP:
-
Cytidine-2′,3′-cyclic phosphate
- CMB:
-
3′-Deoxycytidine-2′-monoborate ester
- 2′-CMP:
-
Cytidine-2′-monophosphate
- 3′-CMP:
-
Cytidine-3′-monophosphate
- γGT:
-
γ-Glutamyl transpeptidase
- HEPES:
-
N-(2-Hydroxyethyl)piperazine-N′-ethanesulfonic acid
- PDB:
-
Protein Data Bank
- RNase A:
-
Ribonuclease A
- Tris:
-
Tris(hydroxymethyl)aminomethane
References
Bertagnolli BL, Hanson JB (1973) Plant Physiol 52:431–435
Gresser MJ (1981) J Biol Chem 256:5981–5983
Moore SA, Moennich DMC, Gresser MJ (1983) J Biol Chem 258:6266–6271
Borah B, Chen CW, Egan W, Miller M, Wlodawer A, Cohen JS (1985) Biochemistry 24:2058–2067
Rehder D, Holst H, Quaas R, Hinrichs W, Hahn U, Saenger W (1989) J Inorg Biochem 37:141–150
Georgalis Y, Zouni A, Hahn U, Saenger W (1991) Biochim Biophys Acta 1118:1–5
Krauss M, Basch H (1992) J Am Chem Soc 114:3630–3634
Wladkowski BD, Svensson LA, Sjolin L, Ladner JE, Gilliland GL (1998) J Am Chem Soc 120:5488–5498
Leon-Lai CH, Gresser MJ, Tracey AS (1996) Can J Chem 74:38–48
Zhang M, Zhou M, VanEtten RL, Stauffacher CV (1997) Biochemistry 36:15–23
Lima CD, Klein MG, Hendrickson WA (1997) Science 278:286–290
Rupert PB, Massey AP, Sigurdsson ST, Ferre-D’Amare AR (2002) Science 298:1421–1424
Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C (1997) J Biol Chem 272:843–851
Sternweis PC, Gilman AG (1982) Proc Natl Acad Sci USA 79:4888–4891
Maruta S., Henry GD, Sykes BD, Ikebe M (1993) J Biol Chem 268:7093–7100
Sondek J, Lambright DG, Noel JP, Hamm HE, Sigler PB (1994) Nature 372:276–279
Fisher AJ, Smith CA, Thoden JB, Smith R, Sutoh K, Holden HM, Rayment I (1995) Biochemistry 34:8960–8972
Dominguez R, Freyzon Y, Trybus KM, Cohen C (1998) Cell 94:559–571
Coureux P-D, Sweeney HL, Houdusse A (2004) EMBO J 23:4527–4537
Smith KW, Johnson SL (1976) Biochemistry 15:560–565
Kim DH, Marbois BN, Faull KF, Eckhert CD (2003) J Mass Spectrom 38:632–640
Kim DH, Faull KF, Norris AJ, Eckhert CD (2004) J Mass Spectrom 39:743–751
Sugiyama M, Hong Z, Whalen LJ, Greenberg WA, Wong C-H (2006) Adv Synth Catal 348:2555–2559
Tate SS, Meister A (1978) Proc Natl Acad Sci USA 75:4806–4809
London RE, Gabel SA (2001) Arch Biochem Biophys 385:250–258
London RE, Gabel SA (2002) Biochemistry 41:5963–5967
Transue TR, Krahn JM, Gabel SA, DeRose F, London RE (2004) Biochemistry 43:2829–2839
Babine RE, Rynkiewicz MJ, Jin L, Abdel-Meguid SS (2004) Lett Drug Des Discov 1:35–44
Raines RT (1998) Chem Rev 98:1045–1065
Herries DG, Mathias AP, Rabin BR (1962) Biochem J 85:127–134
Crook EM, Mathias AP, Rabin BR (1960) Biochem J 74:234–238
Coddington JM, Taylor MJ (1989) J Coord Chem 20:27–38
Baker WR, Kintanar A (1996) Arch Biochem Biophys 327:189–199
Babcock L, Pizer R (1980) Inorg Chem 19:56–61
Hahn U, Desai-Hahn R, Ruterjans H (1985) Eur J Biochem 146:705–712
El Harrous M, Parody-Morreale A (1997) Anal Biochem 254:96–108
Perlman ME, Davis DG, Koszalka GW, Tuttle JV, London RE (1994) Biochemistry 33:7547–7559
Lisgarten JN, Gupta V, Maes D, Wyns L, Zegers I, Palmer RA, Dealwis CG, Aguilar CF, Hemmings AM (1993) Acta Crystallogr Sect D 49:541–547
Zegers I, Maes D, Dao-Thi M-H, Poortmans F, Palmer R, Wyns L (1994) Protein Sci 3:2322–2339
Pizer R, Selzer R (1984) Inorg Chem 23:3023–3026
Weser U (1967) Z Naturforsch B 22:457–458
Chapelle S, Verchere J-F (1988) Tetrahedron 44:4469–4482
Antonov AK, Ivanina TV, Berezin IV, Martinek K (1968) Dokl Acad Nauk SSSR 183:1435–1438
Yonetani T, Theorell H (1964) Arch Biochem Biophys 106:243–251
Transue TR, Gabel SA, London RE (2006) Bioconj Chem 17:300–308
Oki T, Yoshimoto A, Sato S, Takamatsu A (1975) Biochim Biophys Acta 410:262–272
Reddi KK, Dreiling DA (1982) Clin Biochem 15:109–112
Sharp KA, Friedman RA, Misra V, Hecht J, Honig B (1995) Biopolymers 36:245–262
Bauer C-A, Pettersson G (1974) Eur J Biochem 45:473–477
Dreyer MK, Schulz GE (1996) J Mol Biol 259:458–466
Reuter W, Wiegand G, Huber R, Than ME (1999) Proc Natl Acad Sci USA 96:1363–1368
Acknowledgements
The authors are grateful to Yi-chien Lee for providing a preprint of his study of the interaction of 3′-CMP with RNase A. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabel, S.A., London, R.E. Ternary borate–nucleoside complex stabilization by ribonuclease A demonstrates phosphate mimicry. J Biol Inorg Chem 13, 207–217 (2008). https://doi.org/10.1007/s00775-007-0311-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-007-0311-1