Abstract
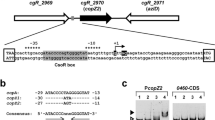
In Enterococcus hirae, copper homeostasis is controlled by the cop operon, which encodes the copper-responsive repressor CopY, the copper chaperone CopZ, and two copper ATPases, CopA and CopB. The four genes are under control of CopY, which is a homodimeric zinc protein, [Zn(II)CopY]2. It acts as a copper-responsive repressor: when media copper is raised, CopY is released from the DNA, allowing transcription to proceed. This involves the conversion of [Zn(II)CopY]2 to [Cu(I)2CopY]2, which is no longer able to bind to the promoter. Binding analysis of [Zn(II)CopY]2 to orthologous promoters and to control DNA by surface plasmon resonance analysis defined the consensus sequence TACAnnTGTA as the repressor binding element, or “cop box”, of Gram-positive bacteria. Association and dissociation rates for the CopY–DNA interaction in the absence and presence of added copper were determined. The dissociation rate of [Zn(II)CopY]2 from the promoter was 7.3×10-6 s-1 and was increased to 5×10-5 s-1 in the presence of copper. This copper-induced change may be the underlying mechanism of copper induction. Induction of the cop operon was also assessed in vivo with a biosensor containing a lux reporter system under the control of the E. hirae cop promoter. Half-maximal induction of this biosensor was observed at 5 μM media copper, which delineates the ambient copper concentration to which the cop operon responds in vivo.








Similar content being viewed by others
Abbreviations
- TCEP:
-
tris(2-carboxyethyl)phosphine
- RU:
-
response units
- TG buffer:
-
50 mM tris-SO4, pH 7.8, 5% (v/v) glycerol
References
Odermatt A, Suter H, Krapf R, Solioz M (1992) Ann NY Acad Sci 671:484–486
Odermatt A, Suter H, Krapf R, Solioz M (1993) J Biol Chem 268:12775–12779
Odermatt A, Solioz M (1995) J Biol Chem 270:4349–4354
Solioz M, Vulpe C (1996) Trends Biochem Sci 21:237–241
Cobine P, Wickramasinghe WA, Harrison MD, Weber T, Solioz M, Dameron CT (1999) FEBS Lett 445:27–30
Strausak D, Solioz M (1997) J Biol Chem 272:8932–8936
Wunderli-Ye H, Solioz M (1999) Biochem Biophys Res Commun 259:443–449
Himeno T, Imanaka T, Aiba S (1986) J Bacteriol 168:1128–1132
Wittman V, Wong HC (1988) J Bacteriol 170:3206–3212
Cobine PA, George GN, Jones CE, Wickramasinghe WA, Solioz M, Dameron CT (2002) Biochemistry 41:5822–5829
Wimmer R, Herrmann T, Solioz M, Wüthrich K (1999) J Biol Chem 274:22597–22603
Rosenzweig AC (2001) Acc Chem Res 34:119–128
Rensing C, Fan B, Sharma R, Mitra B, Rosen BP (2000) Proc Natl Acad Sci USA 97:652–656
Hemmerich P, Sigwart C (1963) Experientia 19:488–489
Solioz M, Waser M (1990) Biochimie 72:279–283
Rogowsky PM, Close TJ, Chimera JA, Shaw JJ, Kado CI (1987) J Bacteriol 169:5101–5112
Stoyanov JV, Magnani D, Solioz M (2003) FEBS Lett 546:391–394
Franke S, Grass G, Rensing C, Nies DH (2003) J Bacteriol 185:3804–3812
Stoyanov JV, Hobman JL, Brown NL (2001) Mol Microbiol 39:502–512
Outten FW, Outten CE, Hale J, O’Halloran TV (2000) J Biol Chem 275:31024–31029
Engohang-Ndong J, Baillat D, Aumercier M, Bellefontaine F, Besra GS, Locht C, Baulard AR (2004) Mol Microbiol 51:175–188
Chan AY, Lim BL (2003) J Mol Biol 333:21–31
Multhaup G, Strausak D, Bissig K-D, Solioz M (2001) Biochem Biophys Res Commun 288:172–177
Changela A, Chen K, Xue Y, Holschen J, Outten CE, O’Halloran TV, Mondragon A (2003) Science 301:1383–1387
Acknowledgment
We thank Kristian Raaby Poulsen and Thomas Weber for valuable experimental help and Christopher Rensing for providing the E. coli CopA-knockout strain DW3110. This work was supported by grant 31-68075.02 from the Swiss National Foundation, and by the International Copper Association (M.S.) and the Deutsche Forschungsgemeinschaft DFG (G.M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Portmann, R., Magnani, D., Stoyanov, J.V. et al. Interaction kinetics of the copper-responsive CopY repressor with the cop promoter of Enterococcus hirae . J Biol Inorg Chem 9, 396–402 (2004). https://doi.org/10.1007/s00775-004-0536-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-004-0536-1