Abstract
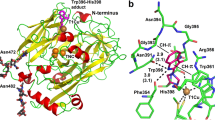
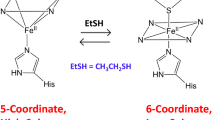
Ferrochelatase, the terminal enzyme in heme biosynthesis, catalyses metal insertion into protoporphyrin IX. The location of the metal binding site with respect to the bound porphyrin substrate and the mode of metal binding are of central importance for understanding the mechanism of porphyrin metallation. In this work we demonstrate that Zn2+, which is commonly used as substrate in assays of the ferrochelatase reaction, and Cd2+, an inhibitor of the enzyme, bind to the invariant amino acids His183 and Glu264 and water molecules, all located within the porphyrin binding cleft. On the other hand, Mg2+, which has been shown to bind close to the surface at 7 Å from His183, was largely absent from its site. Activity measurements demonstrate that Mg2+ has a stimulatory effect on the enzyme, lowering K M for Zn2+ from 55 to 24 µM. Changing one of the Mg2+ binding residues, Glu272, to serine abolishes the effect of Mg2+. It is proposed that prior to metal insertion the metal may form a sitting-atop (SAT) complex with the invariant His-Glu couple and the porphyrin. Metal binding to the Mg2+ site may stimulate metal release from the protein ligands and its insertion into the porphyrin.




Similar content being viewed by others
Abbreviations
- N-MeMP:
-
N-methylmesoporphyrin
- SAT:
-
sitting atop complex
References
Dailey HA, Dailey TA, Wu CK, Medlock AE, Wang KF, Rose JP, Wang BC (2000) Cell Mol Life Sci 57:1909–1926
Al-Karadaghi S, Hansson M, Nikonov S, Jönsson B, Hederstedt L (1997) Structure 5:1501–1510
Wu CK, Dailey HA, Rose JP, Burden A, Sellers VM, Wang BC (2001) Nat Struct Biol 8:156–160
Karlberg T, Lecerof D, Gora M, Silvegren G, Labbe-Bois R, Hansson M, Al-Karadaghi S (2002) Biochemistry 41:13499–13506
Schubert HL, Raux E, Wilson KS, Warren MJ (1999) Biochemistry 38:10660–10669
Leech HK, Raux-Deery E, Heathcote P, Warren MJ (2002) Biochem Soc Trans 30:610–613
Raux E, Schubert HL, Warren MJ (2000) Cell Mol Life Sci 57:1880–1893
Fodje MN, Hansson A, Hansson M, Olsen JG, Gough S, Willows RD, Al-Karadaghi S (2001) J Mol Biol 311:111–122
Schubert HL, Raux E, Brindley AA, Leech HK, Wilson KS, Hill CP, Warren MJ (2002) EMBO J 21:2068–2075
Lavallee DK (1985) Coord Chem Rev 61:55–96
Inamo M, Kamiya N, Inada Y, Nomura M, Funahashi S (2001) Inorg Chem 40:5636–5644
Lecerof D, Fodje M, Hansson A, Hansson M, Al-Karadaghi S (2000) J Mol Biol 297:221–232
Blackwood MEJ, Rush TS, Romesberg F, Schultz PG, Spiro TG (1998) Biochemistry 37:779–782
Franco R, Ma JG, Lu Y, Ferreira GC, Shelnutt JA (2000) Biochemistry 39:2517–2529
Lu Y, Sousa A, Franco R, Mangravita A, Ferreira GC, Moura I, Shelnutt JA (2002) Biochemistry 41:8253–8262
Kohno H, Okuda M, Furukawa T, Tokunaga R, Taketani S (1994) Biochim Biophys Acta 1209:95–100
Gora M, Grzybowska E, Rytka J, Labbe-Bois R (1996) J Biol Chem 271:11810–11816
Ferreira GC, Franco R, Mangravita A, George GN (2002) Biochemistry 41:4809–4818
Franco R, Pereira AS, Tavares P, Mangravita A, Barber MJ, Moura I, Ferreira GC (2001) Biochem J 356:217–222
Hansson M, Al-Karadaghi S (1995) Proteins 23:607–609
Otwinowski Z, Minor W (1997) Methods Enzymol 276:307–326
Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Acta Crystallogr D 54:905–921
Bashford D, Gerwert K (1992) J Mol Biol 224:473–486
Bashford D (1997) In: Ishikawa Y, Oldehoeft RR, Reynders JVW, Tholburn M (eds) Scientific computing in object-oriented parallel environments. Springer, Berlin Heidelberg New York, pp 233–240
Honig B, Nicholls A (1995) Science 268:1144–1149
Pearlman DA, Case DA, Caldwell JW, Ross WS, Ferguson DM, Seibel GL, Singh UC, Weiner PK, Kollman PA (1995) AMBER 4.1. University of California, San Francisco
Bayly CI, Cieplak P, Cornell WD, Kollman PA (1993) J Phys Chem 97:10269–10280
Zheng YJ, Bruice TC (1997) J Am Chem Soc 119:8137–8145
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, revision A.5. Gaussian, Pittsburgh
Sigfridsson E, Ryde U (1998) J Comput Chem 19:377–395
Barone V (1994) Chem Phys Lett 226:392–398
Hehre WJ, Radom L, Scheyer PvR, Pople JA (1986) Ab initio molecular orbital theory. Wiley-Interscience, New York
Schafer A, Horn H, Ahlrichs R (1992) J Chem Phys 97:2571–2577
Jordan PM, Dailey HA (1990) Mol Aspects Med 11:21–23
Dailey HA (1987) Ann NY Acad Sci 514:81–86
Cowan JA (1991) The biological chemistry of magnesium. VCH, New York
Fodje M, Al-Karadaghi S (2002) Protein Eng 15:353–358
Sellers VM, Wu CK, Dailey TA, Dailey HA (2001) Biochemistry 40:9821–9827
Acknowledgements
The authors are grateful to Anders Liljas for helpful discussions. This work was supported by grants from the Swedish Natural Science Research Council (to S.A.) and the Swedish Council for Forestry and Agricultural Research and Carl Tryggers Stiftelse för Vetenskaplig Forskning (to M.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lecerof, D., Fodje, M.N., Alvarez León, R. et al. Metal binding to Bacillus subtilis ferrochelatase and interaction between metal sites. J Biol Inorg Chem 8, 452–458 (2003). https://doi.org/10.1007/s00775-002-0436-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-002-0436-1