Abstract
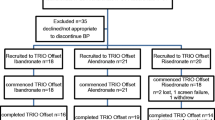
The aims of this study are to investigate changes in serum calcium (Ca) level after switching from either non-therapy, bisphosphonate, selective estrogen receptor modulators (SERM) or teriparatide treatments to a combination therapy of denosumab (DMAb), and eldecalcitol, and the association between early changes in serum calcium and changes in bone metabolic markers and bone mineral density (BMD). 129 patients with postmenopausal osteoporosis (32 non-pretreatment, 50 bisphosphonates, 18 SERM, and 29 teriparatide) were recruited and switched to DMAb plus eldecalcitol. Serum calcium levels, bone metabolism markers, and BMD measurements of the lumbar spine and femoral neck were evaluated. All groups showed an increase in BMD 6 months and 1 year after DMAb administration compared to baseline via suppression of bone metabolism markers. The TPD group showed a significant decrease in serum calcium level 1 week after the first injection of DMAb and eldecalcitol compared to baseline and the bisphosphonate group. Changes in serum calcium level from baseline to 1 week after the first injection of DMAb trended to correlate with changes in bone metabolism markers and lumbar BMD. The risks of DMAb-induced hypocalcemia are different between starting and switching from bone resorption inhibitors and bone formation promoters. Therefore, appropriate assessment before administration of DMAb, including pretreatment therapy as well as serum Ca and bone metabolic markers will help identify the risk of hypocalcemia following DMAb in combination with eldecalcitol. Our findings also showed that early change in serum Ca level after DMAb initiation could potentially predict the efficacy for therapy reaction.





Similar content being viewed by others
References
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733. https://doi.org/10.1007/s00198-006-0172-4
(1997) Who are candidates for prevention and treatment for osteoporosis? Osteoporos Int 7:1–6
Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R (2012) Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat Rev Drug Discov 11:401–419. https://doi.org/10.1038/nrd3705
Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, Hadji P, Hofbauer LC, Alvaro-Gracia JM, Wang H, Austin M, Wagman RB, Newmark R, Libanati C, San Martin J, Bone HG (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24:153–161. https://doi.org/10.1359/jbmr.080901
Brown JP, Roux C, Ho PR, Bolognese MA, Hall J, Bone HG, Bonnick S, van den Bergh JP, Ferreira I, Dakin P, Wagman RB, Recknor C (2014) Denosumab significantly increases bone mineral density and reduces bone turnover compared with monthly oral ibandronate and risedronate in postmenopausal women who remained at higher risk for fracture despite previous suboptimal treatment with an oral bisphosphonate. Osteoporos Int 25:1953–1961. https://doi.org/10.1007/s00198-014-2692-7
Roux C, Hofbauer LC, Ho PR, Wark JD, Zillikens MC, Fahrleitner-Pammer A, Hawkins F, Micaelo M, Minisola S, Papaioannou N, Stone M, Ferreira I, Siddhanti S, Wagman RB, Brown JP (2014) Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone 58:48–54. https://doi.org/10.1016/j.bone.2013.10.006
Ferrari S, Adachi JD, Lippuner K, Zapalowski C, Miller PD, Reginster JY, Torring O, Kendler DL, Daizadeh NS, Wang A, O’Malley CD, Wagman RB, Libanati C, Lewiecki EM (2015) Further reductions in nonvertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 years. Osteoporos Int 26:2763–2771. https://doi.org/10.1007/s00198-015-3179-x
Kamimura M, Nakamura Y, Ikegami S, Uchiyama S, Kato H, Taguchi A (2017) Significant improvement of bone mineral density and bone turnover markers by denosumab therapy in bisphosphonate-unresponsive patients. Osteoporos Int 28:559–566. https://doi.org/10.1007/s00198-016-3764-7
Nakamura Y, Suzuki T, Kamimura M, Ikegami S, Murakami K, Uchiyama S, Taguchi A, Kato H (2017) Two-year clinical outcome of denosumab treatment alone and in combination with teriparatide in Japanese treatment-naive postmenopausal osteoporotic women. Bone Res 5:16055. https://doi.org/10.1038/boneres.2016.55
Farinola N, Kanjanapan Y (2013) Denosumab-induced hypocalcaemia in high bone turnover states of malignancy and secondary hyperparathyroidism from renal failure. Intern Med J 43:1243–1246. https://doi.org/10.1111/imj.12283
Ungprasert P, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Bischof EF (2013) Life-threatening hypocalcemia associated with denosumab in a patient with moderate renal insufficiency. Am J Emerg Med 31:e1–e2. https://doi.org/10.1016/j.ajem.2012.11.011
Anastasilakis AD, Toulis KA, Polyzos SA, Anastasilakis CD, Makras P (2012) Long-term treatment of osteoporosis: safety and efficacy appraisal of denosumab. Ther Clin Risk Manag 8:295–306. https://doi.org/10.2147/TCRM.S24239
Block GA, Bone HG, Fang L, Lee E, Padhi D (2012) A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 27:1471–1479. https://doi.org/10.1002/jbmr.1613
Ishikawa K, Nagai T, Sakamoto K, Ohara K, Eguro T, Ito H, Toyoshima Y, Kokaze A, Toyone T, Inagaki K (2016) High bone turnover elevates the risk of denosumab-induced hypocalcemia in women with postmenopausal osteoporosis. Ther Clin Risk Manag 12:1831–1840. https://doi.org/10.2147/TCRM.S123172
Nakamura Y, Suzuki T, Kato H (2017) Serum bone alkaline phosphatase is a useful marker to evaluate lumbar bone mineral density in Japanese postmenopausal osteoporotic women during denosumab treatment. Ther Clin Risk Manag 13:1343–1348. https://doi.org/10.2147/TCRM.S142828
Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M, Nakamura T (2011) A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures—a randomized, active comparator, double-blind study. Bone 49:605–612. https://doi.org/10.1016/j.bone.2011.07.011
Ebina K, Hashimoto J, Shi K, Kashii M, Hirao M, Yoshikawa H (2015) Undercarboxylated osteocalcin may be an attractive marker of teriparatide treatment in RA patients: response to Mokuda. Osteoporos Int 26:1445. https://doi.org/10.1007/s00198-014-2993-x
Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, Burnett-Bowie SA (2015) Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 386:1147–1155. https://doi.org/10.1016/S0140-6736(15)61120-5
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan 17K10913 (T. Asano).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tsuyoshi Asano, Tomohiro Shimizu, Daisuke Takahashi, Masahiko Takahata, Hiroki Hamano, Masahiro Ota, Shigeto Hiratsuka, Dai Sato, and Norimasa Iwasaki declare that they have no conflict of interest.
Additional information
Tsuyoshi Asano and Tomohiro Shimizu contributed equally to this work.
About this article
Cite this article
Asano, T., Shimizu, T., Takahashi, D. et al. Potential association with early changes in serum calcium level after starting or switching to denosumab combined with eldecalcitol. J Bone Miner Metab 37, 351–357 (2019). https://doi.org/10.1007/s00774-018-0928-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-018-0928-x